le Chatelier’s Principle Statement and Different Effects
Statement of Le Chatelier’s Principle: “When a constraint or stress in a direction is applied to a system in equilibrium, the equilibrium position changes so as to undo the constraint”.
Or
“If a system in equilibrium is altered by changing concentration, pressure, or temperature, the equilibrium shifts in direction that minimize or undo the effect of the change”.
Or
“If a system at equilibrium disturbed by some change, the system will shift so as to neutralize the effect of change”.
Effect of concentration change
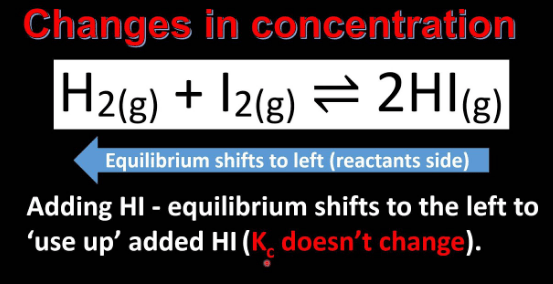
Change in concentration is directly affected by equilibrium. If the concentration of the reactant increase, the equilibrium is disturbed. According to Le-Chatelier’s Principle, equilibrium will shift to the right to keep the value of ‘Kc’ constant and as a result of this more product is formed.
If the concentration of the product increases, the equilibrium is disturbed. According to Le-Chatelier’s principle, equilibrium will shift to the left to keep the value of ‘Kc’ constant, and a result of this concentration of reactant increases.
Effect of Temperature Change on Le Chatelier’s Principle
There are two types of chemical reaction:
- The exothermic reaction, in which heat is evolved and is favored at low temperature.
- The endothermic reaction, in which heat is absorbed and is favored at high temperature.
Le Chatelier’s Principle For Exothermic Reaction
According to principle, if the temperature is decreased in an exothermic reaction the equilibrium shift from forwarding direction, and as a result of this more products are formed. And with the rise in temperature, the equilibrium will shift towards the backward direction and the concentration of the product is decreased.
For Endothermic Reaction
According to the principle, if the temperature is decreased in an endothermic reaction the equilibrium shift from backward direction and a result of this concentration of products is decreasing. And with the rise in temperature, the equilibrium will shift from the forward direction and the concentration of the product is increased.
Effect of Pressure Change
Change in pressure only affects gaseous equilibrium because the change in pressure is related to the change in volume. Pressure has no effect on solids and liquids because they are almost incompressible, hence their volumes are very little affected by the change in pressure.
Effect of Catalyst
A catalyst has no effect on the equilibrium. It increases the rate of reaction by lowering the energy of activation and enables equilibrium to be reached more quickly.
References:
https://en.wikipedia.org/wiki/Le_Chatelier%27s_principle
https://www.chemguide.co.uk/physical/equilibria/lechatelier.html
https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Equilibria/Le_Chatelier’s_Principle
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch16/lechat.html
https://www.cliffsnotes.com/study-guides/chemistry/chemistry/equilibrium/le-chateliers-principle