Difference Between Pure Substance And Mixture
The Basic Difference Between Pure Substance And Mixture is that Pure Substances are always composed of the same kind of particles and are homogeneous in nature with respect of origin while mixtures are variably composed having components that retain their characteristic properties.
Difference Between Pure Substance And Mixture in Tabular Form
Pure Substance | Mixture |
Pure Substances contain only a single type of substance. | Mixtures contain two or more two substances. |
they can be a gas, liquid, or solid. | they only can be homogeneous or heterogeneous substances. |
they cannot be separated into other substances by some physical methods. | they can be separated into other substances by some physical methods. |
hydrogen, pure water, gas, and gold are common examples of pure substances. | sugar, oil, sand, and water are common examples of mixtures. |
due to the single type of substance, the composition remains the same throughout. | due to the many types of substance, the composition is different throughout and cannot be bonded chemically. |
components of pure substances present in a definite or fixed proportion. | components of mixtures present can vary. |
all the elements or compounds in nature are pure substances. | all the HOG and HED are mixtures. |
Pure Substance Vs. Mixture Explanation:
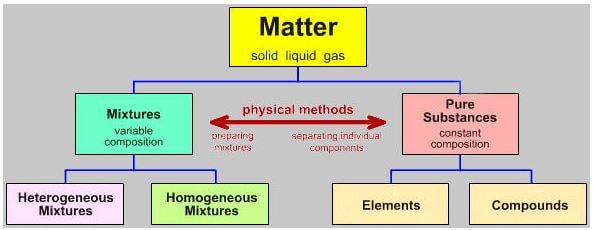
everything present on this earth is known as a matter which can be defined as anything which occupies mass and space is called “matter”. the matter is consists of three fundamental states i.e., solid, liquid, and gas.
apart from the three states of matter, we can classify matter in pure substances and mixtures. substances always have a specified and definite composition that cannot be further subdivided into any state or form.
when we talk about pure substances, they can be divided into elements and compounds. these substances make mixtures. i.e., two or more substances can make up a mixture.
like substances, there are two further types of mixtures such as homogenous mixtures and heterogeneous mixtures. anyways, these are not relevant topics here. we will explain simply the difference between pure substances and mixtures.
What is Pure Substance?
those substances which are made with a single type of atoms or contain only one type of particles having fixed and constant structures are called pure substances. aluminum, iron, silver, and gold are some common examples of pure substances.
a pure substance can be made with only a single solely substance and cannot be isolated into different substances.  they have definite physical and chemical properties. pure substances are grouped into two major categories: compound and elements.
they have definite physical and chemical properties. pure substances are grouped into two major categories: compound and elements.
Elements
elements are those pure substances which contain a single type of atoms which cannot be divided or transform into simpler form or substances by any physical or chemical mode.
for example, if Gold is breaking down it will make Gold again. elements can be metals, no-metals, and metalloids (metalloid is a mixture of metal and non-metal).
Compounds
like elements, compounds are also pure substances with a combination of two or more elements together. they are combined chemically by a fixed proportion.
in contrast, compounds can be broken down by some chemical methods. we can take water as a compound example as water is a compound of two elements hydrogen and oxygen in a fixed proportion 2:1.
Characteristics of compounds:
- compounds are Non-electrolytes.
- Pure substances contain a single type of atoms and molecules and are homogenous in nature.
- having high activation energy, have lower reactions.
- they have a fixed boiling and melting point.
- in compounds, the constituents can easily be separated by some physical methods e.g crystalization and heating.
- the properties of constituent elements will be different from participants.
- having a constant or uniform composition of elements.
- participates in a chemical reaction to form expected products.
- they are generally non-polar or insulable in water.
- their chemical reactions occur slowly.
- can be homogenous or heterogeneous.
Some Examples of Pure Substances
all the elements are said to be compounds approximately. gold, diamond, copper, oxygen, baking soda chlorine, Compounds such as water, salt or crystals, etc. are some common examples of pure substances.
What is a Mixture?
if a substance contains two or more various kinds of matter joined together by some physical method not chemically is called an impure substance. in other words, impure substances are known as mixtures. for example, a mixture of gases, solution of water and salt, solution of water and sugar, etc are called mixtures.
the mixture can be isolated into other substances. they have different chemical and physical properties that depend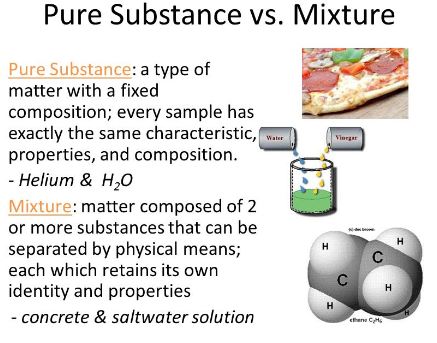 on the pure substance proportions present in the mixture. mixtures are further grouped into two types i.e., Homogeneous and heterogeneous mixtures.
on the pure substance proportions present in the mixture. mixtures are further grouped into two types i.e., Homogeneous and heterogeneous mixtures.
homogeneous mixture:
homogeneous mixtures are solutions having a uniform or unvarying in the configuration in composition. they may contain two or more distinct elements joined together chemically. soda water, a mixture of salt, lemonade are some common examples of a homogeneous mixture.
heterogeneous mixture:
mixtures having nonuniform composition or the composition vary from spot to spot inside a sample of the mixture are called heterogeneous mixtures. for example, a mixture of oil and water, sand and soil, etc. are heterogeneous mixtures.
Characteristics Properties Of Mixtures
- they do not have specified properties
- melting and boiling points may differ.
- have a variable composition.
- formed by a physical change.
Example Of Mixtures
- water and oil solution
- water and sand solution.
- Gas and water liquid
- different gases in the atmosphere.
Conclusion on Difference Between Pure Substance And Mixture:
Organic matter is divided into two major categories:
(1) Mixtures
(2) Pure Substances.
Pure Substances can be categorized into Compounds and Elements. mixtures are made up of different compounds by some physical methods that can be separated into their constituent elements or compounds.
A substance can be composed with one or more same type of elements ( atoms or molecules) while a mixture is composed of different type of elements (atoms or molecules) which are not chemically bonded. mixtures are further divided into homogeneous and heterogeneous mixtures.
You May Also Like: