Difference Between Isotropic And Anisotropic
in minerals, “Isotropic” and “anisotropic” are two contrasting adjectives that show the properties of different materials. The Basic Difference Between Isotropic And Anisotropic is that Isotropic materials are diffuse the same in every direction and characterized by a single diffusion coefficient (D) while Anisotropic materials are diffused differently in any direction. they have characterized by diffusion tensor or 3×3 matrix. they also possess elements of direction with the presence of their descriptions.
In this post, you are going to learn about the Beaker step by step with Diagrams.
This post Also includes:
- An overview
- Isotropic vs anisotropic – Comparison Table
- What is Isotropic?
- What is anisotropic?
- Lots more
So if you want to get benefits from this post you’ll love this post.
Let’s Dive right in…!
Isotropic vs Anisotropic – An overview
The words isotropy and anisotropy are useful in several ways and fields. it depends on how and where we use them. their meaning may slightly be different in several situations. Isotropic and anisotropic terms define the property of matter.
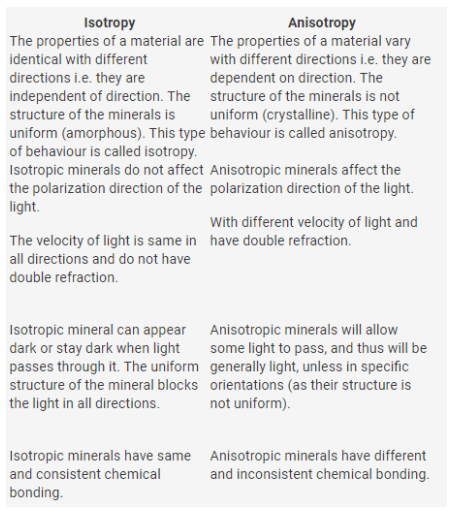
“Anisotropic” refers to the properties of a material present in different directions. A different chemical bond may possess a condition for anisotropy in all directions. on the other hand, a mineral can also be considered anisotropic due to the reason that it allows some light to pass through.
in crystal materials, the arrangement of their atoms is very important and directly affects their mechanical and physical properties. hence, this orientation of atoms leads them to categorize into broadly two classes i.e., isotropic materials and anisotropic materials.
Difference Between Isotropic And Anisotropic in Tabular Form
Isotropic | Anisotropic |
Properties | |
They possess invariable properties and are independent of the direction. | They possess variable properties and are dependent on direction. |
Characteristics | |
They have no special Characteristics | Dichroism, Optical activity, birefringence, and scattering are some common characteristics of Anisotropic. |
Refractive index | |
Shows single refractive index | Show more than one refractive index. |
A consistent Chemical Bond is present. | The chemical bond is inconsistent. |
Applications | |
Used for lasers and windows. | Commonly used in Wedges, Polarizers, and Waveplates. |
Examples | |
A Cup of Tea | Wood shows the pattern like Anisotropic. |
Sunlight | |
The sunlight cannot pass through them. | The sunlight can penetrate and shines them. |
Appearance in Nature | |
Appeared as Dark in Nature | Appeared as Bright in Nature. |
Double Refraction | |
Double refraction is uncommon in isotropic | It is common in anisotropic. |
Speed of Light | |
Light Penetrates at equal speed in all directions. | Light speed can vary in different directions. |
Conduction of Electricity | |
Electricity conducts the same in all directions. | Conduction of electricity may different in different directions. |
Symmetry | |
Shows a cubic symmetry | Symmetry is not cubic in anisotropic. |
Radiation Intensity | |
Same as in all directions | Different in multiple directions. |
What is isotropic?
the word isotropic refers to the chemical and physical properties of the material in all directions. in other words, they do not depend on the direction or constant in all directions. one can find the amorphous materials in the composition of isotropic materials. the chemical bonding also shows a constant behavior in them.
this behavior can be shown easily in cell walls of plant cells that reflect the isotropic material characteristics in all directions. on the other hand, sodium salts and rock sals are also isotropic of cubic crystals. they both show invariable properties. independent of the conditions of solids, the hardness, and solidity of cubic crystals remain the same in all directions. because the optical properties are dependent on direction hence they are not present there. the sodium salt, rock salt, and glass are common examples of isotropic.
What are the Characteristics of Isotropic?
- highly dependent on the direction.
- the electricity can conduct as same in all directions of the materials.
- they have a cubic symmetry.
- they are not capable of double refraction.
- the radiation strength remains the same in all directions.
- does not capable of dispersion effect.
- no optical activity and dichroism effect present in them.
- they have a dark presence in the landscape.
- they cannot pass the sunlight through them.
- also, the speed of light remains the same in all directions or orientations.
- their composition is said to e amorphous.
What are the Applications of Isotropic?
- commonly used in laser lights.
- can be seen on windows.
- used for isotropic radiators.
- mostly used in making a reference for the antenna.
What is Anisotropic?
the Anisotropic also refers to the chemical and mechanical properties that may vary by changing their orientation, place, or position. in other words, mainly they dependent on the orientation of crystals.
they are variable in different directions which have composite material composition. The internal cytoplasm of the cells represents the anisotropic manner because of having continuously changed physical and chemical properties.
their dimensions also vary from place to place or in all directions due to the presence of intercellular organelles in the cytoplasm. wood, gypsum, and cubic crystals are considered good examples of Anisotropic.
Try Also: Difference Between Chemical and Physical Properties
What are the Characteristics of Anisotropic?
- they have independent properties from place to place or in different directions.
- the cubic symmetry does not exist in them.
- radiation force varies in different orientations.
- the conduction of electricity also varies in all directions.
- possess multiple refraction indexes.
- the optical activity also presents in them.
- the double refraction is also very commonly seen in anisotropic crystals.
- both dichroism effect and dispersion effect also present in them.
- they are very bright in nature.
- sunlight can travel through them.
- they are composite materials in the composition.
- the speed of light may vary in all different dimensions.
Applications of Isotropic
- can be used in optical waveplates.
- used for wedges.
- they are also used in electronic materials.
- polarizers are made by using anisotropic crystals.
- many types of magnetic materials are made with them.
You May Also Like: