Chemistry
What is the Difference between Evaporation and Boiling?
the major difference between Evaporation and Boiling is that Evaporation is a process in which the water changes its state from liquid to vapor without boiling while boiling is the process in which a substance changes its state from liquid state to gaseous state.
What is Evaporation?
What is Boiling?
difference between Evaporation and Boiling
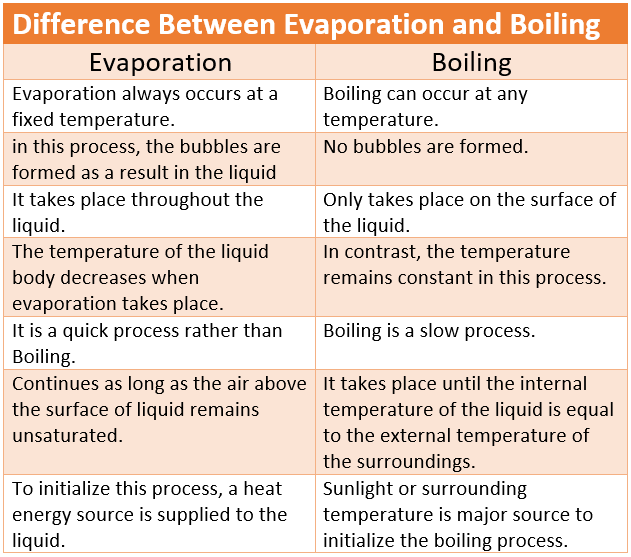
| Evaporation | Boiling |
| Evaporation always occurs at a fixed temperature. | Boiling can occur at any temperature. |
| in this process, the bubbles are formed as a result of the liquid. | No bubbles are formed. |
| it takes place throughout the liquid. | only takes place on the surface of the liquid. |
| the temperature of the liquid body decreases when evaporation takes place. | in contrast, the temperature remains constant in this process. |
| It is a quick process rather than Boiling. | Boiling is a slow process. |
| continues as long as the air above the surface of liquid remains unsaturated. | it takes place until the internal temperature of the liquid is equal to the external temperature of the surroundings. |
| to initialize this process, a heat energy source is supplied to the liquid. | sunlight or surrounding temperature is a major source to initialize the boiling process. |
| the temperature remains constant to get the required results. | temperature can vary depends on the situation. |
| Evaporation reached the boiling point. | Evaporation can harm you at serious consequences. |
| known as the process of vaporization of liquids at room temperature. | known as the process of evaporation of liquids at the boiling point of liquids. |
| sweat from our bodies is one of the examples of evaporation. | example, water boils at 100 °C. |


dogshit. actually wrong in basic concepts science solved a couple centuries ago. actually factually incorrect
the content is WRONG please correct that it has confused many students
The concepts are totally wrong . Please correct it
This difference is not correct. Boiling process is alway fast than evaporation. In boiling bubbles are formed but bubbles and not formed in evaporation. Please post only correct informations on website and don’t post this type of false informations again.
Thanks….
What the hell is this, If u can’t give right guidance then atleast don’t misguide students. These no. of mistakes are just unacceptable