Gay Lussac’s Law: Formula and Definition
What is Gay Lussac’s Law
Joseph Gay Lussac in 1802, established the pressure-temperature law which is called Gay Lussac’s law also known as Amontons’s law.
This law states that “The pressure of the fixed mass of the gas is directly proportional to Kelvin temperature at constant volume.”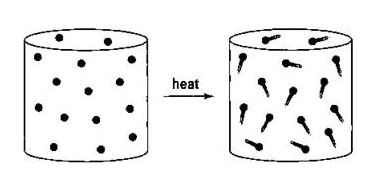
P ∝ T ( when ‘V’ and ‘n’ are constant )
P = kT
P/T = k
in other words, the pressure of a given mass of the gas with the absolute temperature of that gas by keeping constant volume.
At more than one pressures and temperature for the same quantity of the same gas,
so the gay lussac’s law formula is :
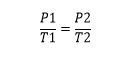
Gay-Lussac established Pressure Law which states that the pressure of an enclosed gas is directly proportional to the temperature of that gas and he was incorrect. he also credited for his relationship about fixed mass pressure and temperature by keeping the volume of gas constant. the following laws are regarded as Pressure Law or Amontons’s law and Dalton’s law respectively.
Molar Volume:
It follows from Avogadro’s law that one mole of any gas at a given temperature ‘T’ and pressure ‘P’ has the same fixed volume. In order to compare the molar volume of gases, reference temperature and pressure have been fixed which is 0°C and one atm pressure. It is found experimentally that one mole of any gas at S.T.P. occupies a volume of 22.414dm3.