Standard Hydrogen Electrode (SHE) : Definition Applications and diagram
What is Standard Hydrogen Electrode?
Standard Hydrogen Electrode (SHE) is hydrogen electrodes consists of a platinum plate immersed in the 1 molar solution of the sulphuric acid. A current of pure hydrogen gas is passed continuously through the solution under the presence of 1-atm.
The platinum absorbs hydrogen gas on its surface and the platinum-coated with the hydrogen behaves as if it were made entirely of hydrogen.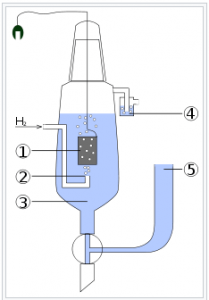
Since absolute electrode potential cannot be measured, hence it is determined by comparing with the hydrogen electrode which is a reference electrode. The arbitrary hydrogen electrode has assigned a potential of 0.000 volts.
Electrode Potential
The difference of the potential created between a metal and the solution of its salt is called electrode potential. OR it is the measure of the tendency of an electrode to lose or gain electrons. It is denoted by ‘E0’.