Chemistry
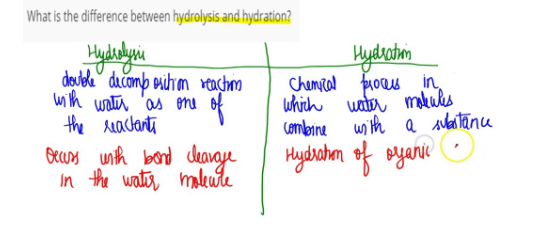
What is the difference between the terms hydrolysis and hydration?
Difference Between Hydration and Hydrolysis is that the addition of a molecule of water to increase hydrogen and oxygen count is called hydration while the addition of a molecule of water in an attempt to convert to the complex structure to a simpler one is known as hydrolysis.
Hydration
- The surrounding of the solute molecules by solvent molecules (water molecules) is called hydration.
- It is a physical process.
- pH remains constant.
Hydrolysis
- the reaction of cat-ion, an-ion or both with water so, as to change its pH is called hydrolysis.
- It is a chemical process.
- pH either increases or decreases.