Tyndall effect
In this post, you are going to learn about the Tyndall effect or Willis–Tyndall scattering step by step with Diagram.
This post includes:
- A Step by step Guide about Tyndall effect in Chemistry with a diagram
- the Experiment in colloidal Suspension
- Lots of Real-life Examples
- Lots more
So if you want to get benefits from this post you’ll love this post.
Let’s Dive right in…
An Overview – Definition
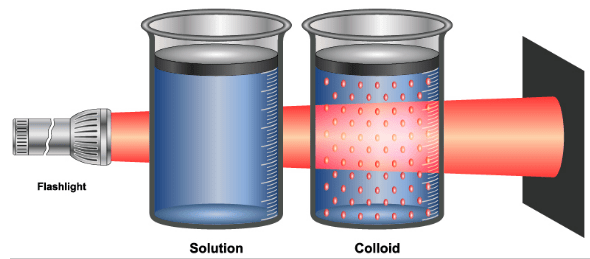
the Tyndall effect or Willis–Tyndall scattering is a phenomenon used for light scattering in a colloid by using different particles or as a light beam. it can be used in a very fine colloidal suspension. blue color smoke from motorcycle engines is a common example. named because of a physicist John Tyndall after the 19th century.
According to Willis–Tyndall, longer wavelengths tend to more transmission and shorter wavelengths are likely to diffused reflection or transmission by the help of scattering. the amount of scattering highly dependant on the frequency and density of light particles involved in the experiment. the effect can only be visible when light-scattering particulate matter is dispersed and the diameter of the medium’s particle should be in the range between 40 and 1000 nanometers in diameter. which is approximately near to the human visible light.
most applications are found in fine suspensions and colloidal solutions or mixtures for example in nephelometers to find the density of particles and their sizes in aerosols. when using Rayleigh scattering, blue light scattered strongly compares to red with Tyndall effect.
Common Examples of Tyndall Effect:
- two-stroke engines of motorcycles leave the blue color of smoke which is a common example of the Tyndall Effect.
- flashlight beam into a glass of milk shines is due to this effect. to make this effect visible, add some dilute water in the milk so that you can clearly feel colloidal particles.
- some gases are dispersed in a liquid dispersing medium to make foams, for example, shaving creams.
- It is also used in lab settings and commercial purposes to find out the aerosols particle size.
- The visible beam of car or any other vehicle’s headlights in fog.
- the scattering causes Blue eye color because the eye’s iris have a translucent layer over them.
- The glass Opalescent appears blue with Tyndall effect that shines through it appears orange.
Comparison with Rayleigh Scattering
most of the people are unable to understand the Difference Between Rayleigh and Tyndall effect. for example, the blue color of our sky is also due to the scattering of light and is called Rayleigh because the particles molecules are smaller in the air than a colloid while the shining of the dust particle is due to the following effect because their particles are too large to scatter the light.
Watch an Exclusive Video
You May Also Like: