What is the Difference Between Compound And Mixture?
Difference Between Compound And Mixture is that compound is the mixture of two or more than two elements and is always homogeneous in nature while a mixture is formed when elements or compounds just mix together to form a mixture and can be homogenous or heterogeneous. in mixtures, no new compound is formed.
What is a Compound?
A compound is usually makeup by a mixture of two or more separate elements by chemical bonding. there are two known types of compounds e.g. ionic compounds and covalent compounds. the elements in a compound are always present in a fixed ratio.it is a pure matter.
Examples of Compounds
we can see many examples of compounds in our daily life. they are the essential part for the survival of human beings e.g. water is a compound of two elements say hydrogen and oxygen. some of the examples are given below.
- Water
- Baking Powder
- Table Salt
- Mouthwash
- Nailpolish Remover
- Toothpaste
- Sugar
- glucose
- Soap
What is a Mixture?
A mixture is a substance made up of the mixing of other substances or compounds together. they can be separated easily into their constituent elements. all the elements present in the mixture are distinct. their boiling and melting points are not constant.
11 Common Examples of Mixtures
- The mixture of salt and paper
- the mixture of sand and water
- suspensions
- Colloids
- liquids
- gases
- solids
- the mixture of sugar and salt
- soda
- air
- Alloys
identify two common solutions
A solution is simply a mixture of two different substances together. one of them is known as the solvent and the other is solute. in the solution of water and sand, the sand is solvent while water is solute.
how are the atoms in a compound held together?
the compound atoms are held together with the help of chemical bonding. bonding can be present in either an ionic bond or a covalent bond. the other property of atoms takes part is the attraction between the molecules of the substances which is known as cohesion.
Properties of Compounds
compounds are usually formed by the combination of either two or more different elements. their properties always vary from their constituent elements and present in a fixed ratio. they can be separated by physical methods like distillation and filtration. compounds are separated by chemical methods.
Properties of Mixtures
Mixtures are formed by when elements or compounds are mixed with each other. their properties may vary from their constituent compounds. they are not enabled to show their own characteristics. mixtures or separated simply by physical methods. Always present as pure matter.
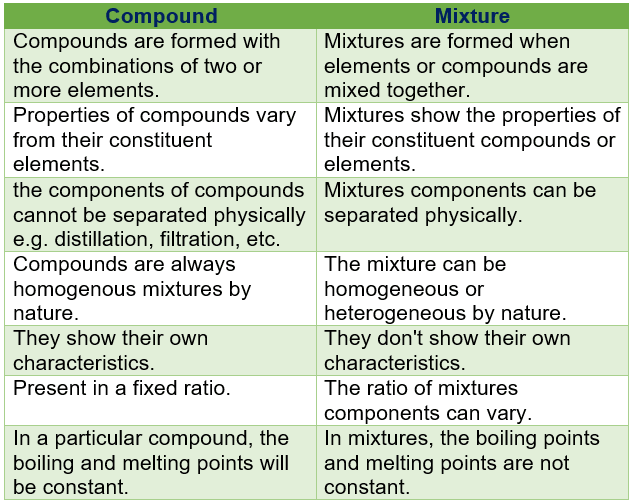
Difference Between Compound And Mixture in Tabular Form
| Compound | Mixture |
| compounds are formed with the combinations of two or more elements. | Mixtures are formed when elements or compounds are mixed together. |
| properties of compounds vary from their constituent elements. | mixtures show the properties of their constituent compounds or elements. |
| the components of compounds cannot be separated physically e.g. distillation, filtration, etc. | mixtures components can be separated physically. |
| compounds are always homogenous mixtures by nature. | the mixture can be homogeneous or heterogeneous by nature. |
| they show their own characteristics. | they don’t show their own characteristics. |
| present in a fixed ratio. | the ratio of mixtures components can vary. |
| in a particular compound, the boiling and melting points will be constant. | in mixtures, the boiling points and melting points are not constant. |
| energy is given out to compounds through a chemical reaction. | mixtures do not or very little amount of energy transfers. |
| The compound is pure Matter. | the mixture is an impure matter. |
| compounds are formed by absorption or of heat, light, or electrical energy. | formation of mixtures may not need absorption or evolution of light, heat, or electrical energy. |
| their constituents can only be separated by chemical methods. | their constituents can only be separated by physical methods. |
| in compounds, the constituents combine in a fixed ratio. | the constituents of mixtures may present in any ratio to form a new mixture. |
Conclusion:
The difference between a Compound and a Mixture is that a Compound is a pure substance containing only one kind of molecule and the ratio of components is fixed while the mixture is not a pure substance, it may consist of various types of atoms and molecules. the ratio of components may vary.
You May Also Like: