Classification of Elements based on s,p,d and f Orbitals
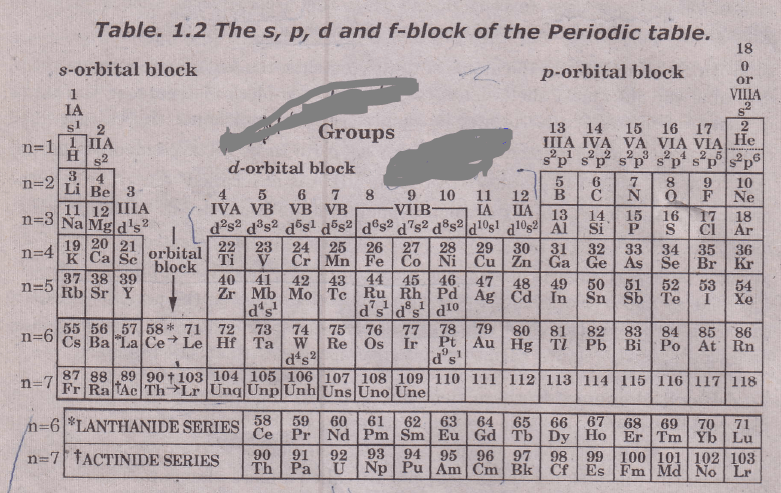
the long form of the periodic table divides the elements into four major blocks known as s, p, d, and f. this division represents the name of the orbital and received from the last electron of the shell.
s block elements are those in which their last electron enters into their outer most shells. p block elements are those in which last electrons enter in anyone from the last three outermost shells respectively and in d Block elements, electrons enter in anyone five d orbitals while in f block elements, electrons enter in any one of the 7 f orbitals.
The modern periodic table sets out the elements in the order, in which successive energy levels in the atoms are being filled by electrons.
Since the electrons occupy specific energy levels and subshells, thus there is a definite relationship between the periodic table and filling of the subshell.
[wp_ad_camp_1]
On the basis of the filling of electronic subshells, the elements may be classified into four sections or blocks i.e., s-, p-, d- and f-block elements in the periodic table depending upon the nature of the atomic orbitals into which the differentiating (last) electron enters.
For the purpose of this classification of elements, only the orbital of the highest energy into which the last electron goes is considered. This orbital may not always belong to the outermost shell.
Classification of Elements
- s-Block elements: in the atoms of these elements, the differentiating (last) electron enters the ns orbital of the outermost shell which is being progressively filled. Consequently, the valence shell electronic configuration of these elements varies from ns1 to ns2. The elements of groups IA, IIA, H and He belong to this block since the valence shell configuration of group IA and H is ns1 and group III and He is ns2.
The s-block elements lie on the extreme left of the periodic table and consist of active metals (except H and He). The properties of s-block elements depend on the number of electrons present in ns orbital. - p-Block elements: In the atoms of these elements, the last electron enters the p-orbital of the outermost shell, hence they are called p-block elements. In the atoms of these elements, the ns orbital is completely filled and hence the valence shell configuration of these elements varies from ns2p1 to ns2p6.
The elements of groups IIIA, IVA, VA, VIA, VIIIA and VIIIA (Group VIIIA is also called “zero groups”) are the members of this block, since the valence—shell configurations are ns2p1, ns2p2, rs2p3,ns2p4, ns2p5, and ns respectively. These elements lie at the extreme right of the periodic table and consist of some metals, non-metals, metalloids, and inert gases.
[wp_ad_camp_2]
The s- and p-block elements (i.e., A group elements ) in the periodic table are called the representative or main group elements. The elements generally show distinct and fairly regular variation in their properties with atomic number.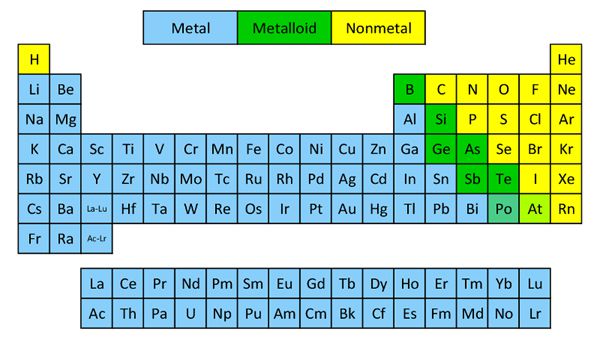
- d-Block elements: In these elements, either in their atomic state or in any of their oxidation state the last electron enters the (n—1)d orbital of the penultimate (i.e., n—1) shell. In other words, in these elements (n 1)d orbital is being progressively filled. Hence these elements are called d-block elements.
The electronic configuration for the two outer shells of these elements can be represented by (n—1) d1-10ns1-2. With the exceptions of Cr, Cu, Nb, Mo, Ru, Rh, Pd, Ag, Pt, and Au, in the atoms of these elements, the ns-orbital is completely filled (n2 configuration). These elements are placed in the middle of the periodic table, between s- and p-block elements. Elements in the B Groups (except LLB) in the periodic table are also called d-transition elements or more simply as transition elements or transition metals.[wp_ad_camp_3]These elements are all metals and are characterized by electrons being added to d-orbitals. The transition elements are classified into four series corresponding to the filling of 3d-, 4d-, 5d- and 6d-orbitals of the (n-1). the shell of these atoms as follows:
1st Transition Series (3d series): 21Sc through 29 Cu. In the atoms of these elements, the last electron enters the 3d orbitals.
2nd Transition Series (4d series). 39Y through 47Ag.
3rd Transition Series (5d series). 57La and 72Hf through 9 Au.
4th Transition Series (6d series). (incomplete) 89Ac and elements 104 through 112. These are the elements of the 7th period (incomplete period).
[wp_ad_camp_4]
Strictly speaking, the Group IIB elements, Zn, Cd, and mercury are not d-transition metals since their “last” electron goes into s-orbitals; but they are usually discussed with the d-transition metals because their chemical properties are similar.
4f-block elements (inner Transition elements). In these elements, either in their atomic state or in any of their common oxidation states, the last electron enters the (n-2)f orbitals of the (n-2) shell. In other words, in these elements ‘(n—2)f orbital is being 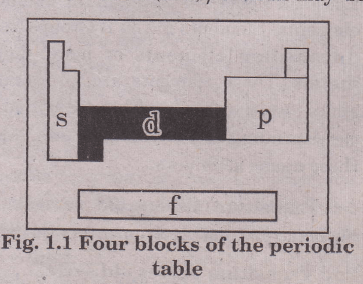 progressively filled. Hence these elements. (n-2) f-orbitals may be either 4f orbitals or 5f orbitals. The inner transition elements are located between Group IIIB and IVB in the periodic table and are usually shown in two separate rows below the main part of the body. The f—subshell has a maximum population of 14 electrons, so there are 14 lanthanides and 14 actinides. The f—block elements are of two types.
progressively filled. Hence these elements. (n-2) f-orbitals may be either 4f orbitals or 5f orbitals. The inner transition elements are located between Group IIIB and IVB in the periodic table and are usually shown in two separate rows below the main part of the body. The f—subshell has a maximum population of 14 electrons, so there are 14 lanthanides and 14 actinides. The f—block elements are of two types.
4f-Series or 1st Inner Transition Series (Lanthanides): This series has 14 elements: 58Ce through 71Lu.
5f-Series or 2nd Inner Transition Series (Actinides): 90Th through 103Lr. All of the actinides are radioactive and from neptunium (element 93) on, are synthetic. They have been produced in nuclear reactors or by using particle accelerators.