Bond Energy : definition formula and examples
“The amount of energy required to break a bond between two atoms in a diatomic molecule is known as bond energy”.
SI Unit of Bond Energy
Bond energy is expressed in kilojoules per mole (KJ/mol) unit.
Factors Affecting Bond Energy
Bond energy depends on the following factors:
Polarity of Bond
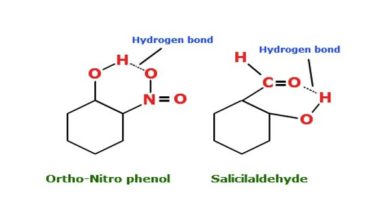
Bond energy is the measure of the strength of bonds. Since polar covalent bonds are the stronger bond than non-polar covalent bonds due to the presence of ionic character. Therefore, bond energies of polar covalent bonds are greater than the pure covalent bonds (non-polar bond).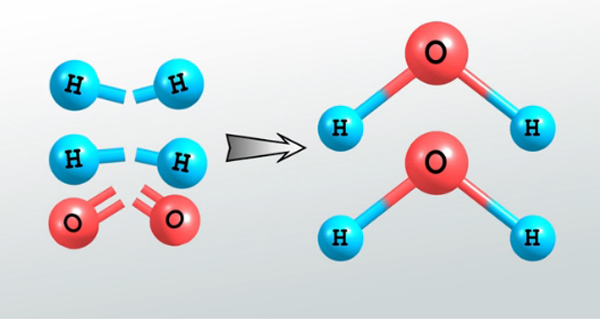
Bond Distance
The value of bond energy also depends upon the bond distance. The shorter the bond distance, the stronger the bond and greater would be the bond energy.
For example, triple bonds are usually shorter than double bonds which in turn are shorter than the single bonds. Hence, the bond energies for the multiple bonds would generally greater than those of single bonds.