Types of Indicators with Uses and Examples in Chemistry
A chemical indicator is used to slow down the chemical reaction by changing its color until the reaction either  finished or reached its equilibrium point. . Indicators are complex molecules that are themselves weak acids (e.g. phenolphthalein) or weak bases (e.g. methyl orange).there are six major types of Indicators in Chemistry. they are organic compounds.
finished or reached its equilibrium point. . Indicators are complex molecules that are themselves weak acids (e.g. phenolphthalein) or weak bases (e.g. methyl orange).there are six major types of Indicators in Chemistry. they are organic compounds.
They have different colors in acidic and alkaline solutions. various specific functions are fulfilled by the use of different types of indicators. Litmus is a common indicator. It is red in the acidic solutions and blue in the alkaline solutions. which are as follows:
- thymol blue
- bromothymol blue
- methyl orange
- phenolphthalein
- litmus
- bromcresol green
Each indicator has a specific color in the acidic medium which changes at a specific pH to another color in the basic medium. For example, phenolphthalein is colorless in the strongly acidic solution and red in the strongly basic solution. It changes color at a pH of about 9. This means phenolphthalein is colorless in a solution with a pH of less than 9. If the pH is above 9, phenolphthalein is red.
Types of Indicators
- Acid-Base Indicator
- Natural acid-base indicators
- Indicators of oxidation-reduction reactions (redox)
- Adsorption (precipitation) indicators
- Metallochromic or complexometric indicators
- Chemical indicators with luminescence capacity
- Chemical moisture indicators
- Chemical indicators of sterilization
Characteristics of a chemical indicator
while you choose an indicator, it should have met some characteristics and a series of requirements for appropriate use. the requirements are as follows:
- it must not react with substances and should have pure chemically.
- must not degrade in the whole reaction and should remain chemically inert.
- when the reaction reaches the equilibrium point or ends the indicator must have noticeable coloration.
- the Degree of physical integrity may not alter by making it thicker.
Choice of Indicator
For strong acid and weak base, methyl orange is used. For a strong base and weak acid, phenolphthalein is commonly chosen.
Use and Applications of indicators and types of Indicators
Indicators are commonly used to find out the endpoint of acid-base titration.
| Indicators | Colour in Acid | Colour in Base | Colour When Neutral |
| Litmus | Red | Blue | Purple |
| Methyl Orange | Red | Yellow | Orange |
| Phenolphthalein | Colorless | Pink | Colorless |
| Universal Indicator | red | purple | Green |
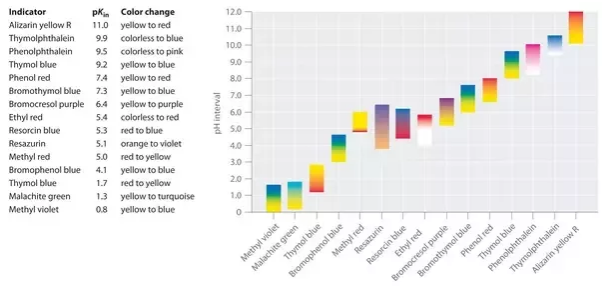
20 Examples of Indicators
- Methyl Violet
- Azolythmine
- Leucomalachite green
- Methyl red
- Thymol Blue
- Bromocresol Green
- Methyl Yellow
- Methyl orange
- Bromophenol Blue
- Congo Red
- Bromocresol Purple
- Eriochrome Black
- Bromothymol Blue
- Alizarin Yellow
- Phenol Red
- Thymolphthalein
- Neutral Red
- Phenolphthalein
- Naphtholphthalein
- Cresol Red
Explanation of Indicators Behavior (types of Indicators)
The indicator dissociates in a solution and gives an ion that has a different color from the un-dissociated molecule of the indicator.
Example
Consider the example of the methyl orange, which is red-colored in unionized form and yellow-colored in unionized form.
If we represent an indicator by the general formula ‘HIn’ then the dissociation of the indicator can be shown as follows:
HIn ====== H+ + In–
The behavior of Indicator (Methyl Orange)
- In Acidic Medium
If an acid is added which increases the concentration of the H+ then equilibrium will move to the left and unionized ‘HIn’ will predominate and the solution will be red.
In Basic Medium
If a base is added which reduces the concentration H+ then equilibrium will move to the right and ionized ‘In’ will predominate and the solution will be yellow.
List of important chemical indicators
Eriochrome Black: It is a bluish-black and complexometric powder indicator that is used for complexometric titrations and added to the Aliquot for getting the dark purple color of the solution. the most common use of that indicator is to determine the hardness of water or complexometric determination of water hardness. it consists of calcium carbonate CaCO3. when all calcium is dissolved in water titration, the indicator shows an electric blue color.
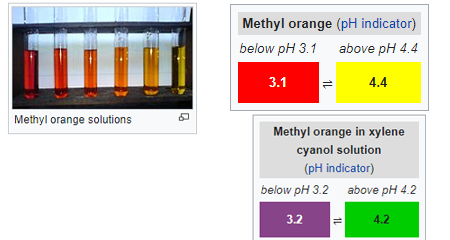
Methyl Orange(MO): it is the most important and very common azo dyes or PH indicator for Acid-Base volumetry that is often used with acid to determine the base in Aliquot. it is a frequently used titration indicator in various industries such as paper, printing, textile industry, etc. a large quantity is wasted and discharge in industrial water waste. at a high range of PH, it shows orange coloration.
by changing the PH values, also shows distinct color variance. although methyl orange also knows as a universal indicator it does not show the full spectrum of color range and have a very sharp endpoint. despite the excessive use, it has some mutagenic properties hence one should avoid direct contact due to hazardous substances.
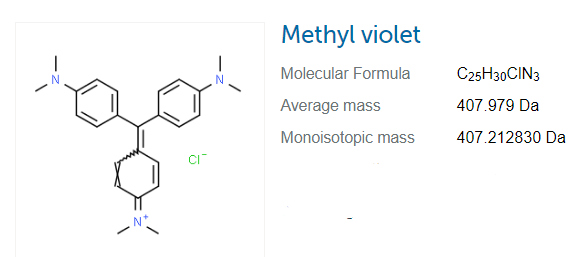
Methyl Violet: methyl violet is a family (also known as gentian violet) of acid-base indicators that belong to organic compounds and extensively used for dyes. it turns into a yellow color at a low PH value of methyl violet. the PH value varies from 0.0 to 2.0 for a blue-violet color. for methyl groups attached to that indicator, we can alter the color of the dye with ease.
the different colors of methyl violet are used for different purposes for example, purple for textiles and deep violet for ink and paint. silica gel also includes a hydration indicator. it is also useful for medical purposes.
Phenolphthalein: another important Acid-Base volumetry indicator having formula C20H14O4. it is a chemical compound that belongs to the phthalein family and can also be written as “Hin” or “PHPH” as short notation. it is used as a laboratory reagent and is a colorless indicator having a PH range of 8.5 which shows pink to dark red by increasing PH above 9.0. being titrated, used for determining acid in aliquot.
Thymol Blue: thymol blue is an acid-base indicator which is also known as thymolsulfonephthalein and used for PH indicator. it is a crystalline powder having a reddish-brown or brownish-green color with two vires or transitions (Acidic PH and Basic PH). by changing the PH value from 1.2 to 2.8, it turns into yellow color in the first transition. while in the second transition, the PH value varies from 8.0 to 9.6 which turns it into blue.
thymol blue is considered a universal indicator that also has some toxic properties that can cause some irritations. hence, it is needed to be used safely. it is also very dangerous if swollen, unfortunately. it can also cause hazardous consequences when using the above 10% value.
Bromophenol Blue: bromophenol blue also is known as (tetrabromophenolphthalein) and is used as a common PH indicator. at low PH, shows yellow color while at PH 3.0 to 4.6 appear as purple.
Congo Red: Congo Red is an organic compound and known as a common Acid-Base indicator that turns into the blue-violet color of solutions at low PH value. it turns into Red at PH 3.8 to 5.0 which also known as Azo dye. its molecular formula is C32H22N6Na2O6S2 having a 696.7g/mol molecular weight. Congo Red was discovered by Paul Bottinger in 1883 in Germany. Congo Red is also known for water-soluble in Colloidal Solution.
Methyl Red: methyl red is an acid-base indicator having chemical formula C15H15N3O2 and molar mass 269.304g/mol. it is also called C.I Acid Red 2 that shows Red color at a low PH value and turns yellow at PH 4.4to 6.2. it is a dark red crystalline powder and also known as Azo dye.
Bromocresol Green: Bromocresol Green or BCG is a triphenylmethane dye and is considered an acid-base indicator that shows yellow color at low PH. it also turns into a blue-green color when changing PH value between 3.8 to 5.4. it is widely used for titration purposes, microbiological growth and DNA agarose gel, electrophoresis. the BCG has chemical formula C21H14Br4O5 and molecular weight 698g/mol. it is highly soluble in benzene and water.
Measuring the pH of a Solution (types of Indicators)
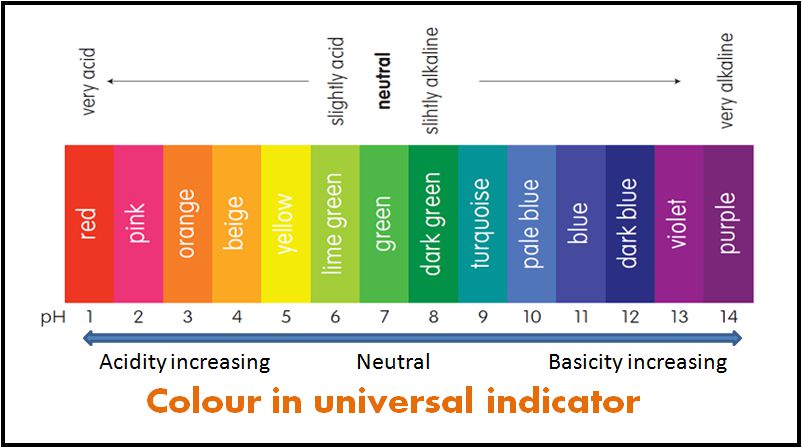
Universal Indicator
Some indicators are used as mixtures from types of Indicators. The mixture indicators give different colors at different pH values. Hence, it is used to measure the pH of a solution. Such a mixed indicator is called a universal indicator or simply a pH indicator.
The pH of a solution can be measured by dipping a piece of Universal Indicator paper in the solution. The pH is then found by comparing the color obtained with a color chart.
The pH Meter
The pH of a solution can be measured with a pH meter. It consists of a pH electrode connected to a meter. The electrode is dipped into the solution and the meter shows the pH either on a scale or digitally. It is a much more reliable and accurate method of measuring pH than the Universal Indicator paper, though the latter is often more convenient.
FAQ’s (Frequently Asked Questions)
What are the different types of indicators?
input, output, outcome, and impact are four different kinds of indicators that use commonly used in laboratories.
What are the 3 types of indicators?
lagging, leading, and coincident are three basic types of indicators in economics.
What are the four types of indicators?
the four types of indicators are as follows: (1) input indicators (2) output indicators, (3) outcome indicators, and (4) impact indicators.
How many types of indicators are there in chemistry?
there are two types of indicators are found in chemistry: (1) Natural Indicators (2) Artificial Indicators
What are two examples of indicators?
Red Cabbage, Cherries, Turmeric are examples of natural indicators while phenolphthalein, methyl orange, and litmus paper are examples of synthetic indicators.
What is the most common indicator?
in the laboratory apparatus, litmus paper is known as the most common indicator which is made from litmus that is extracted from the lichens.
What are universal indicators give example?
Methyl red (4.8 – 6.0), Bromothymol blue (6.0 – 7.6), Thymol blue (8.0 – 9.6) are some examples of universal indicators.
You May Also Like:

Why you have any given example of types of indicator .
Sorry I mean that there are no example of type of indicators
I’ll add some of the examples of indicators soon.