5 States of Matter: properties of solids liquids and gases
What are the three states of matter?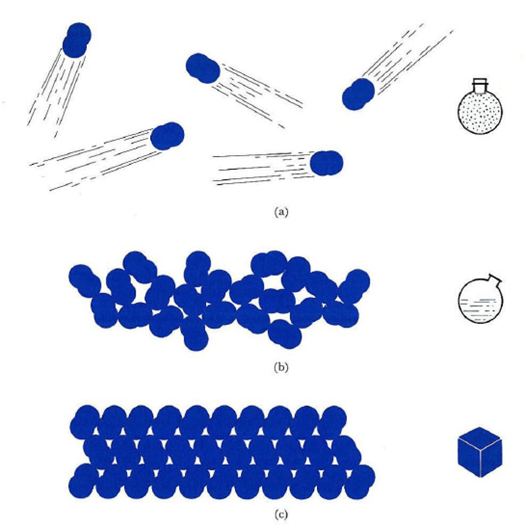
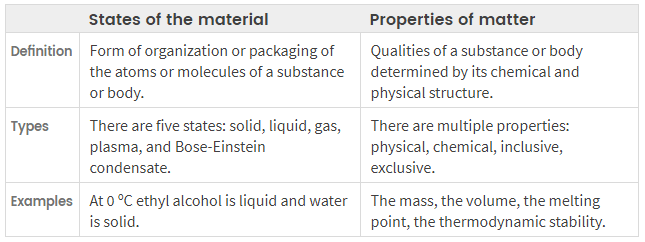
There are five possible states of matter i.e., solid, liquid, gas, plasma, and Bose-Einstein condensate. The simplest form of matter in the gaseous state and most of the matter around us in the solid-state. all the states of matter are present in specific patterns in such a way that their constituent elements are grouped.
While the properties of matter are said to be the set of qualities that are present in any form of matter and they characterized it.
so, the matter can be defined as:
anything in the universe that has mass and occupies space is known as matter. matter consists of very small particles known as atoms or molecules which are, in turn, matter.
states of matter is a way in which matter’s constituents molecules are organized which is also known as an aggregation of states of matter. matter exists in five common states: the fourth and fifth state is rare.
- Gas
- Liquid
- solid
- Plasma
- Bose-Einstein condensate
Gas
A state of matter that has no definite shape as well as no definite volume is called gas: it occupied all the space containers in which it is kept. their atoms and molecules are allowed the freedom to move anywhere in space.
Examples of gases
air, which is a mixture of gaseous elements such as oxygen, nitrogen, and carbon dioxide.
Liquid
A state of matter that has a definite volume but no definite shape is called liquid. it occupies or attains the shape of the container in which it is kept. rather than solids, liquids molecules are grouped together in such a way that they can move freely.
Examples of liquid
- water at room temperature
- oil
- mercury
- alcohol
- glycerine
- Greece
Solid
A state of matter that has both definite shape and volume is called solid. the molecules are tightly packed, which causes them to limit their movements in molecules.
Liquids are less common than solids, gases, and plasmas. The reason is that the liquid state of any substance can exist only within a relatively narrow range of temperature and pressure. the same substance may exist in three states. for example, water in the form of ice is solid; in the form of steam is gas, while a usual form of water is liquid.
Common Examples of Solids
- rocks
- metals
- wood
- Water at temperatures below 0ºC
Plasma
Plasma is the 4th state of matter which is produced when a gas receives a large amount of energy which releases its electrons from outermost shells. it is neither has a definite shape nor volume.
Common Examples of plasma
it is found in:
- stars
- fluorescent lights
- rays
- neon signs and gases
Bose-Einstein condensate
by cooling a group of atoms at absolute zero (-273 ° C), Bose-Einstein condensate can be achieved. practically not moving relative to each other at this stage of atoms, behaving as if they were a single atom.
Common Examples of Bose-Einstein condensed state
- Many of the experiments use laser-cooled rubidium atoms.
it is the inter-molecular distances or spaces between the molecules, which makes difference among the three states. in solids, the molecules are closely packed with one another and have minimum inter-molecular distance. in gases, the molecules are free from one another and have a very large inter-molecular distance. in liquids the situation is intermediate. they have large inter-molecular distance as compared to solids but much less than gases.
inter-molecular distance depends upon the following two opposing factors:
- inter-molecular attraction
- kinetic energy
What are the properties of matter?
properties of matter are those qualities of matter than dependant on the physical structure and chemical composition. these properties are of 4 types i.e, chemical properties, physical properties, intensive and extensive properties.
What are Physical properties?
the physical properties of mater are simply measurable qualities that do not modify the chemical structure of matter. they involve examples such as boiling point, Elasticity, volume, and temperature, etc.
What are Chemical properties?
the qualities of matter that bring up changes to their chemical structure are called chemical properties. reactivity, flammability, toxicity, and thermodynamic stability are general examples of such type.
What are the Intensive properties?
Intensive properties are those which do not depend on the quantity of matter. For example, the density of a material does not change even if we have 1 kilogram or 10 grams of the same material.
What are Extensive properties?
They are those physical properties that change when modifying the amount of matter, such as, for example, volume and mass.
General Terms related to properties of matter as examples
The different properties of matter serve to identify and classify materials.
Specific heat
Specific heat is an intensive physical property that indicates the amount of heat required to increase the temperature by one degree centigrade of a kilogram of material. For example, the specific heat of gold is 129 Joules / ºC per kg, that of sodium chloride is 864 Joules / ºC per kg.
Chemical stability
The ability of matter to react under certain conditions determines its chemical stability. We have, for example, the noble gases (helium, neon, argon, krypton, xenon, radon, and Hoganson) which are the least reactive elements in the periodic table. On the other hand, the alkali metals (lithium, sodium, potassium, rubidium, cesium, and francium) react strongly in the presence of water.
Electric charge
Electric charge is the physical property that determines the interaction forces of matter in an electromagnetic field. There are positive, negative, or neutral charges. Like charges oppose and different charges attract.
Malleability
The ease with which you can transform the material into sheets without breaking it is a physical property known as malleability. For example, coal is not malleable, because when struck with sufficient force it breaks into pieces. Instead, an ounce of gold (28.35 gr) can be spread on a 91 m 2 sheet.
Let us look at the general properties of gases, liquids, and solids. The kinetic molecular theory of gases can help us understand their properties.
Properties of gases
- Gasses don`t have a definite volume and occupy all the available space. The volume of a gas is the volume of the container.
- They don`t have a definite shape and take the shape of the container just like liquids.
- Due to low densities of gases, as compared to those of liquids and solids, the gases bubble through liquids and tend to rise up.
- Gases can diffuse and effuse. This property is negligible in solids but operates in liquids as well.
- Gases can be compared by applying pressure because there is largely empty space between their molecules.
- Gases can expand on heating or by increasing the available volume. Liquids and solids, on the other hand, do not show an appreciable increase in volume when they are heated.
- When the sudden expansion of gases occurs, it is called the Joule Thomson effect.
- Molecules of gases are in a constant state of random motion. They can exert a certain pressure on the walls of the container and this pressure is due to the number of collisions.
- The intermolecular forces in gases are very weak.
Properties of liquids
- Liquids don`t have a definite shape but have a definite volume. Unlike solids, they adopt the shape of the container.
- Molecules of liquids are in a constant state of motion. The evaporation and diffusion of liquid molecules are due to this motion.
- The densities of liquids are much greater than those of gases but are close to those of solids.
- The space among the molecules of liquids is negligible just like solids.
- The intermolecular attractive forces in liquids are intermediate between gases and solids. The melting points and boiling points of gases, liquids, and solids depend upon the strength of such forces.
- Molecules of liquids possess kinetic energy due to their motion. Liquids can be converted into solids on cooling i.e., by increasing their kinetic energy. Molecules of liquids collide among themselves and exchange energy but those of liquids cannot do so.
Properties of solids
- The particles present in solid substances are very close to each other and they are tightly packed. Due to this reason, solids are non-compressible and they cannot diffuse into each other.
- There are strong attractive forces in solids that hold the particles together firmly and for this reason, solids have definite shape and volume.
- The solid particles possess only vibrational motion.
You May Also Like: Difference between Solid Liquid and Gas
Unit of pressure
The pressure of air that can support 760 mmHg column at sea level, is called one atmosphere. It is the force exerted by 760 mm or 76 cm long column of mercury on an area of 1cm2 at 0˚C. It is the average pressure of the atmosphere at sea level 1mmHg = 1 Torr. The S.I. unit of pressure is Nm-2 . one atmospheric pressure i.e. 760 torrs is equal to 101325 Nm-2.
1 pascal = 1Nm-2. Sp, 760torr = 101325Pa= 101.325 kilopascals (Kpa is another unit of pressure)
The unit pounds per square inch ( psi) is used most commonly in engineering work, and 1 atm =760 torr = 14.7 pounds inch-2. the unit millibar is commonly used by meteorologists.
Watch this Video about the States of Matter
References:
https://www.diferenciador.com/estados-de-la-materia-y-propiedades-de-la-materia/