Chemistry
What is the Difference Between Orbit and Orbitals?
The Major Difference Between Orbit and Orbitals is that orbit is a well defined circular path followed by revolving electrons around the nucleus while orbital is a region of space around the nucleus of an atom where the electron is most likely to be found.
Read Also: Difference Between Atomic Orbital And Molecular Orbital
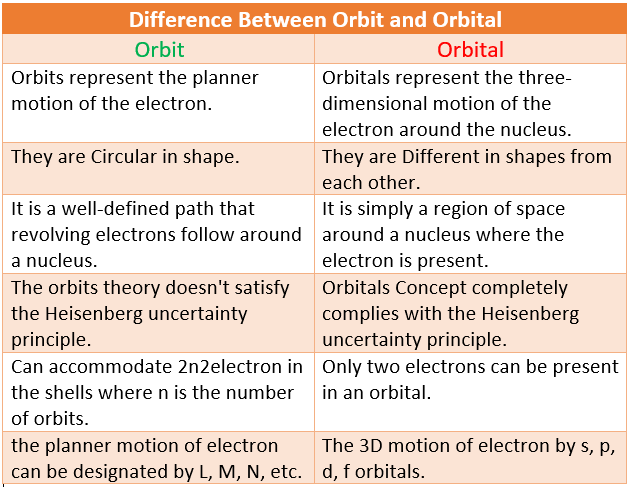
Difference Between Orbit and Orbitals in Tabular Form
| Orbit | Orbital |
| Orbits represent the planner motion of the electron. | orbitals represent the three-dimensional motion of the electron around the nucleus. |
| They are Circular in shape. | they are Different in shapes from each other. |
| it is a well-defined path that revolving electrons follow around a nucleus. | it is simply a region of space around a nucleus where the electron is present. |
| the orbits theory doesn’t satisfy the Heisenberg uncertainty principle. | Orbitals Concept completely complies with the Heisenberg uncertainty principle. |
| Can accommodate 2n2 electron in the shells where n is the number of orbits. | only two electrons can be present in an orbital. |
| the planner motion of electron can be designated by L, M, N, etc. | The 3D motion of electron by s,p,d,f orbitals. |
| According to Bohr’s atomic model | Against the Bohr’s Atomic Model. |

