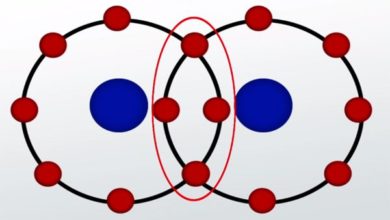
The Major Difference Between Atomic Orbital And Molecular Orbital is that Atomic Orbital occurs around a single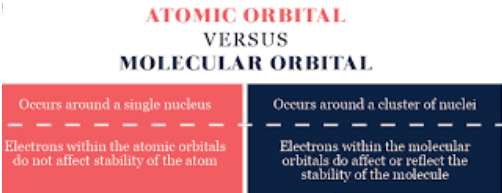 nucleus and electrons within the atomic orbitals do not affect the stability of atoms while Molecular Orbital occurs around a cluster of nuclei and electrons in molecular orbitals affect or reflect the stability of the molecule.
nucleus and electrons within the atomic orbitals do not affect the stability of atoms while Molecular Orbital occurs around a cluster of nuclei and electrons in molecular orbitals affect or reflect the stability of the molecule.
Atomic Orbital
- It consists of a single nucleus.
- The electrons are mono-centric i.e., under the influence of one nucleus.
- Atomic orbitals are represented as s, p, d, and f.
Molecular Orbital
- It consists of more than one nucleus.
- Electrons are polycentric i.e., under the influence of more than one nucleus.
- Molecular orbitals are represented as σ, σ*, etc.
You May Also Like: