Density: Uses and Factors Affecting
What is the density?
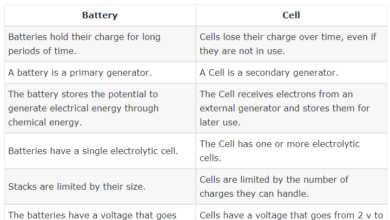
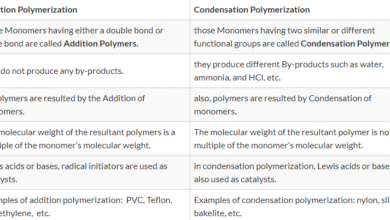
Density is one of the properties of matter. When we talk about the density of matter, we mean the relationship between the mass of matter and the space taken by that mass (size), which determines the density of the material, the size of the atoms in the material, its mass, Of different sizes and different in mass are different density,
for example: if we have a cube made of copper material, and another cube made of wood material, both have the same size, the density of copper cube will be greater than the density of wood cube; Larger than the mass of wood
Law of Density
In order to calculate the density of an object, we need to know the mass of the material and the size of the material. The density is equal to the mass divided by the volume.
According to the law (w = k / h), we need to know two variables of the equation to calculate the third variable (W), and the other form of the law to calculate the mass of the material (K = W x H), but to calculate the size of the material we use the other image of the law (H = K / S);
W: density of the material. K: Block material. v: Size of the material.
Permission: density = mass/volume, and symbols: w = k/h.
Factors Affecting Density
According to the density law mentioned above, we deduce that the density depends on size and mass. The size is variable, so the density changes because the relationship between them is reversed. The smaller the volume the greater the density (while the mass remains constant).
Pressure: The particles of the body get closer to each other the more pressure on them because the compressed body reduces its size, and as the size is inversely proportional to the density and pressure, the greater the pressure on an object increases its density (a relationship between density and pressure).
heat: If an object is heated, its particles move and are separated from each other, thus increasing its volume. As the volume is inversely proportional to the density and uniform with the heat, the heat is inversely proportional to the density.
If the researcher can determine the pressure and temperature affecting a substance, it can determine its density, the materials are less invasive because the molecules are separated from each other, and thus the size and increase the intensity of the fact that the relationship between the opposite,
liquid materials are the highest density of gaseous substances, Solid is the highest density of liquid and gaseous, and the reason is that their molecules are close together, and thus their size decreases and increases automatically.
Uses of Density
One of the most used uses of density is to know how the materials interact when mixed with each other. For example, wood is floating on the surface of the water because it is less dense, whereas if we bring a bar of iron it drowns in water because its density is higher.
The buoys that are placed in the water tanks also float on the surface water is less dense than air.
Another example: when you drive to the car mechanic to check the oils, the mechanic will put some oils in the scale of the hydrometer.
This measure contains several things to calibrate the oils and float some of them in the oil, Those oils, and this is facilitated Some of the batteries produce a chemical when the acid meets the lead.
The density of the solution decreases. When this density is measured, We can find out the remaining amount of charges that battery, and the density of many uses also in physics, engineering, geology, and weather.
How to Calculate Density of any substance?
Here are some examples of how to calculate the density of a substance:
Example 1: If you know that we have an equilateral cube made of plastic, weighs 50 g, and its length is 4.5 cm, what is its density?
The size of the cube = cube size = length of the cube = 3 cubit length = 4.5 cm, and the application of the law: cube size = 4.5 cm 3 = 91.125 cm 3 Now the size and mass are known, Density = mass / volume, density = 50 g / 91.125 cm 3; then density of plastic cube = 0.548 g / cm 3.
Example 2: Aluminum cube, lengths of ribs (6 cm, 4 cm, 4 cm), weighs 400 g, find its density?
Solution: cube size = (height x width x height) cube size = 6 cm * 4 cm * 4 cm cube size = 144 cm 3. Block of the cube = 400 g. Cube density = mass / volume. Density of the cube = 400 g / 144 cm 3. Density of the cube = 2.7 g / cm 3.
Example 3: Parallels made of ice with a density of 0.88 g / cm 3, with a base area of 6.25 cm 2, and a mass of 23.65 g, find its height?
Solution: Base space = length * width = 6.25 cm 2 (given in question).
In order to find the height we first find the size of the parallel rectangles;
where: w = k/h, and since the mass and density is given to us in question, the law applies directly: Size = mass / density = 23.65 /0.88 = 26.875 cm 3. Height = size / area of the base = 26.875 cm / 6.25 cm height of parallel rectangles = 4.3 cm.