Chemistry
Difference between Electronegativity and Electron Affinity
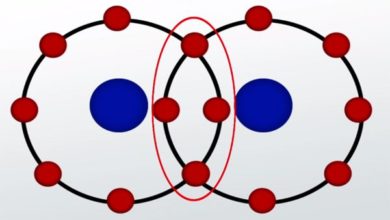
The Major Difference Between Electronegativity And Electron Affinity is that Electronegativity is a chemical property that decides the tendency of an atom to attract electron in a covalent bond, for example, Cl-F while Electron Affinity is the amount of energy that an atom exerts when an electron is added to neutral atom to make it into negative ion i.e. Cl+ e- → Cl-.
electron affinity describes the release of energy to the surrounding while electronegativity is the tendency of an atom to attract the shared pair of electron.
| Electronegativity | Electron affinity |
| It is the tendency of an element to attract shared pair of electron towards itself. | It is the minimum amount of energy released when a gaseous atom absorbs an electron from the surroundings. |
| It has no unit. | It has a unit. |
| It helps in predicting the nature of bond form between atoms. | It helps in predicting either a chemical substance oxidizing or reducing agent. |


This really answered my problem, thank you!