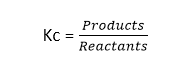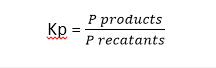Chemistry
Difference Between Kc and Kp
What is Kc?
- It is the equilibrium constant which is a ratio of the concentration of reactants at the equilibrium in a reversible reaction.
- Mathematically,
What is Kp?
- It is the equilibrium constant which is a ratio of partial pressure of products and partial pressure of reactants at the equilibrium in a reversible reaction.
- Mathematically,