Difference Between Ideal Gas And Real Gas
When we want to describe the behavior of gases under different conditions, Ideal gases, and real gases are two major types of gases. An ideal gas obeys all laws of gases under all conditions (temperature & pressure). Another aspect is, it also obeys the ideal Gas Equation PV=nRT that indicates ideal gas is a theoretical gas in which temperature, pressure, and volume are always directly proportional while the number of molecules remains constant.
On the other hand, Real gases obey all gas laws only at low temperatures and pressures. They do not obey the ideal Gas Equation (PV=nRT) but obey the wander Waals equation. They always tend to deviate from ideal gas behavior. Their properties are dependent on the structure of molecules, temperature, and pressure. We can only predict the behavior of real gases through complex mathematical models or experiments.
Note that, ideal gases have point masses occupying certain volumes and are considered to be non-interacting. We can predict their behavior using the ideal gas law under the equation (PV=nRT).
In this post, you are going to learn about the Difference Between Ideal Gas And Real Gas step by step with Diagrams.
This post also includes:
- An overview
- What is The ideal gas?
- what is Real gas?
- Lots more
So if you want to get benefits from this post you’ll love this post.
Let’s Dive right in…!
An overview
Gas is a matter of low density that does not have its own volume or format but rather adapts to the bowl, container, or conical flask where it is kept. Gases are made up of atoms and molecules that interact with each other through intermolecular forces and occupy a finite volume.
By the principles of pressure, volume, and a temperature it is possible to distinguish the real gas from the ideal gas.
What is The ideal gas?
The ideal or perfect gas is from the group of theoretical gases whose randomly moved point particles do not interact with each other, such as oxygen, hydrogen, and carbon dioxide; likewise, it considers inversely proportional changes between pressure and volume, but directly proportional changes of both, pressure and volume, concerning temperature.
The ideal gas is a theoretical concept that we use for our study purposes. For gas to be ideal, it must have the following characteristics. If one of these is missing, then the gas is not considered.
On the other hand, the thermodynamic behavior of real gases does not follow the ideal gas equation, since it maintains high pressure and low temperature, that is, it has very high-density values.
According to the kinetic theory of gases, the behavior of an ideal gas is based on two premises: the gas molecules are not punctual and the interaction energy is not negligible.
In natural gas, in the ideal gas model, the substances are always in gaseous mode. More complex is the behavior of the real substance, which can undergo a phase or state change.
This phase change makes the isotherms of the real gas more complex than those of the ideal gas.
- The intermolecular forces between gas molecules are negligible.
- Gas molecules are considered point particles. Therefore, compared to the space that gas molecules occupy, the volumes of the molecules are negligible.
Normally gaseous molecules fill any given space. Therefore, when a large space is occupied by air, the gas molecule itself is very small compared to space. Therefore, assuming gas molecules as point particles is correct to some extent. However, there are some gas molecules with a considerable volume. Ignoring the volume gives errors in these cases.
According to the first assumption, we have to consider that there is no intermolecular interaction between gas molecules.
However, in reality, there are at least weak interactions between them. But, gaseous molecules move quickly and randomly. Therefore, they do not have enough time to make intermolecular interactions with other molecules.
Therefore, when looking at this angle, it is valid to accept the first assumption as well. Although we say that ideal gases are theoretical, we cannot say that they are 100% true. There are some occasions when gases act like ideal gases. it is characterized by three variables, pressure, volume, and temperature. The following equation defines the ideal gases.
PV = NRT = NkT
- P = absolute pressure
- V = volume of gas
- n = number of moles present
- N = number of molecules present
- R = universal gas constant of the gas
- T = absolute temperature
- K = Boltzmann’s constant
Although there are limitations, we determine the behavior of gases using the above equation.
what is Real gas?
A real gas cannot be compressed indefinitely, contrary to the ideal gas hypothesis. At low pressures, and under the same circumstances, real gases are more compressible than ideal gases, but this happens when their pressure values are higher, which depends on the temperature and the type of gas.
Under normal conditions, when its chemical formula is simple and its relativity is low, the pressure and temperature of real gases will match those of ideal gases, such as helium.
The properties of real gas cannot be explained by the ideal gas law; To understand them, the effects of compressibility, the specific heat capacity, the thermodynamic effects of non-equilibrium, and the Van der Waals forces must be taken into account.
When we talk about the Van der Waals forces, the name of the Dutch scientist who won the Nobel Prize in Physics in 1910 and investigated gases, we refer to the repulsive and attractive forces that exist between molecules and that are very small in the case of real gases.
When one of the two or both assumptions given above is not valid, those gases are known as real gases. We actually find real gases in the natural environment. A real gas varies from the ideal condition to very high pressure. This is because, when very high pressure is applied, the volume where the gas is filled becomes very small.
Then, compared to space, we cannot ignore the size of the molecule. Also, ideal gases reach the real state at very low temperatures. At low temperatures, the kinetic energy of gas molecules is very low. Therefore, they move slowly.
Because of this, there will be intermolecular interaction between gas molecules, which we cannot ignore. For real gases, we cannot use the ideal gas equation above because they behave differently.
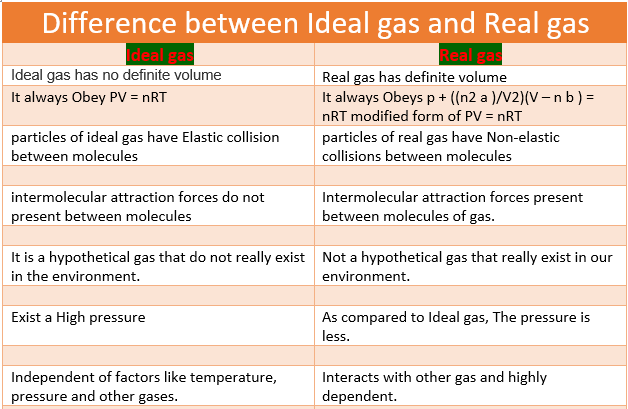
Difference between real gas and ideal gas in Tabular Form | |
Ideal gas | Real gas |
It has no definite volume | It has a definite volume |
It always Obeys PV = NRT or all gas laws under all conditions of temperature and pressure. | It always Obeys p + ((n2 a )/V2)(V – nb ) = nRT modified form of PV = NRT or gas laws at low pressure and high temperature. |
particles of ideal gases have Elastic collision between molecules. | particles of real gases have Non-elastic collisions between molecules. |
molecules volume that is occupied can be negligibly on comparing the total volume. | molecules volume that is occupied can’t be negligibly on comparing the total volume. |
intermolecular attraction forces do not present or are negligible between molecules. | Intermolecular attraction forces present and cannot be negligible between molecules of gas. |
they Have no definite mass. | they have a definite mass. |
It is a hypothetical gas that does not really exist in the environment. | Not a hypothetical gas that really exists in our environment. |
The volume is zero. | The volume is non-zero. |
Exist in High pressure. | As compared to the Ideal, The pressure is less. |
attractive or repulsive forces do not exist between particles. | small attractive or repulsive forces are present between particles. |
Independent of factors like temperature, pressure, and other gases. | Interacts with other gas and is highly dependent. |
Possess only kinetic energy. | Possess both kinetic and potential energy. |
they do not really do not exist. | Nitrogen, Oxygen, and Carbon dioxide are common examples of real gas. |
According to the scientist, the difference between real gases and ideal gases is that one corrects the volume and the other modifies the pressure. It maintains that real gases, at pressures and temperatures close to ambient, act as ideal gases.
Gas is one of the states in which matter exists. It has contradictory properties of solids and liquids. Gases have no order, and they occupy any given space. Its behavior is greatly affected by variables such as temperature, pressure, etc.
We can define Van der Waals’ Law as an equation of state about gas pressure, the universal gas constant, the volume that the gas occupies, and its temperature in absolute value.
The equations of real gases must be applied when measuring the behavior of a gas that differs from the usual conditions of an ideal gas.
These equations show that real gases do not have the infinite expansion, because if they did they could not occupy a greater volume.
Finally, the ideal gas equation works well when the intermolecular attraction of the gas is negligible and the molecules do not occupy a significant part of the total volume, which is true when the pressure is low and the temperature is high.
In other situations, the ideal gas law could give different results. But what is clear is that gases do not always behave like ideal gases.
- For an ideal gas the variable “z” is always worth one, on the other hand, for a real gas, “z” has to be worth different than one.
- The equation of state for a gas dispenses with the variable “z” since this for an ideal gas is worth one. And for a real gas, since this variable has to be different from one, so the formula looks like this: PV = znRT.
- The van der Waals equation differs from those of ideal gases by the presence of two correction terms; one corrects the volume, and the other modifies the pressure.
- Real gases, at pressures and temperatures close to ambient, act as ideal gases.
You May Also Like: