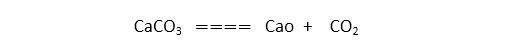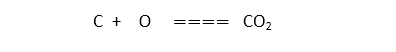What is the difference between endothermic and exothermic reactions?
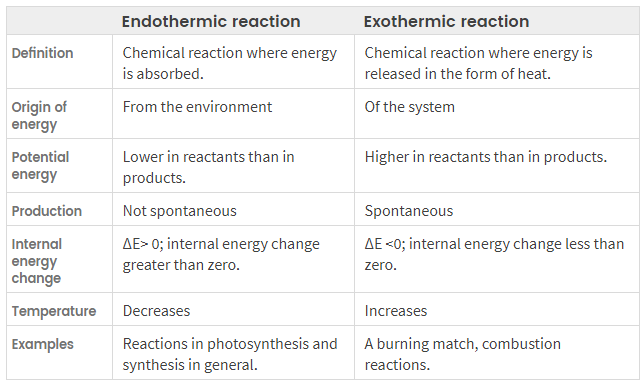
the major difference between endothermic and exothermic reactions is that an endothermic reaction is a process in which energy is acquired from its surroundings in the form of heat while an exothermic reaction is a process in which energy is released to the surroundings in the form of heat. in short, endothermic reactions always require energy while exothermic reactions release energy. both reactions are classified as a reactant or as a product with the participation of energy.
In this post, you are going to learn about endothermic and exothermic reactions step by step with Diagrams.
- An overview
- Differences Table
- What are Endothermic Reactions?
- What are Exothermic Reactions?
- Lots more
So if you want to get benefits from this post you’ll love this post.
Let’s Dive right in..
An Overview
energy is used to produce heat and it has the ability to do work and can be expressed in many forms such as heat, sound, and light, etc. on the other hand, all the chemical reactions contain arrangements involving atoms of different substances that can break or make the chemical bonds. by bringing the change in the energy system could result in the breaking of the chemical bond.
the energy changes occur in the form of temperature, as a result, the temperature or energy of the system and the environment also changes and we can say that energy has been transferred as heat. this heat transfer can be classified into two kinds of reactions i.e., endothermic reaction and exothermic reaction.
An endothermic reaction possesses a process in which hear or energy is acquired from its surroundings while an endothermic process gains energy from its surroundings.
What are Endothermic Reactions?
The word “endothermic” is derived or abbreviated from two Greek word endon which means ” inside” and therme which means “heat”. when a reaction absorbs energy from the environment, in the form of heat it is known as an endothermic reaction. it is a reaction that reactions do not proceed spontaneously. that means, If the environment does not supply heat, the reaction does not occur.
it changes the reaction vessel from hot to cold by absorbing heat from the surrounding environment and lower the temperature of the surrounding. so, the heat is transferred from the outside to the inside of the system.
by placing a thermometer in the presence of the endothermic reaction, the temperature always drops.
in other words, the bond-breaking energy of the endothermic reaction reactants is greater than the total bond formation energy of the products. Furthermore, it is the energy that is needed to start the reaction process and it is always less than the energy released.
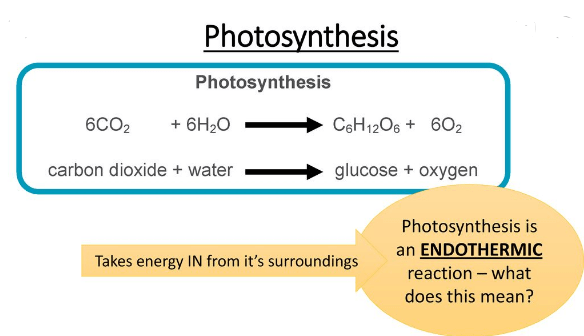
for example, when we dissolve ammonium chloride in water, the glass cools down due to the energy absorption from the outside environment. the photosynthesis process is also a common example of an endothermic reaction in which the sunlight provides the required energy as heat to produce necessary glucose from carbon dioxide and oxygen.
the photosynthesis reaction products (glucose and oxygen) have a greater amount of potential energy as compared to that of reactants i.e., carbon dioxide and water.
the common properties of such type of reactions are as follows:
- A reaction in which heat is absorbed is called an endothermic reaction.
- These are shown by the positive sign of Δ
- These are non-spontaneous reactions.
- The decomposition of reaction is endothermic reactions.
Examples of the endothermic reactions
- Cooking food: Although it may not seem like it, the process of cooking food is endothermic. In order to consume certain foods, we must provide heat.
- Instant cold bag: Cold packs used to treat bumps or sprains are filled with water, but when they are shaken or knocked, a capsule containing ammonium nitrate breaks inside. Mixing ammonium nitrate with water is an endothermic reaction, which causes the bag to cool.
- Photosynthesis: As a tree grows, it absorbs energy from the environment to break down CO2 and H2O.
- Evaporation: Perspiration cools a person down like water extracts heat by changing it into a gas.
- Cooking an egg: Energy is absorbed from the pan to cook the egg.
What are Exothermic Reactions?
The word “exothermic” is formed by exo which means “outward” and thermes, which means “heat”. an exothermic reaction is a process that releases energy to the surroundings, usually in the form of heat. in other words, energy flows out of the system.
This energy is released as heat, so placing a thermometer in the reaction system increases the temperature. Furthermore, energy can also be released in other forms, such as sound, light, etc. Since energy is released during the reaction, the products contain less energy than the reactants.
Therefore, the enthalpy change (∆H) becomes negative. Exothermic reactions can occur spontaneously and, in some cases, be explosive, such as the combination of alkali metals and water.
External energy supply is not necessary for exothermic reactions as they produce the required energy as the reaction proceeds.
However, to start the reaction, an initial energy supply may be necessary.
In this type of reaction, energy is released during bond formation. If the total bond-forming energy is greater than the bond-breaking energy during the reaction, then it is exothermic. If energy is released as heat, the surrounding temperature rises, so the reaction can sometimes be explosive.
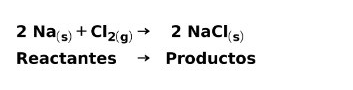
In a chemical reaction, reactants are the compounds that transform and give rise to products. For example, when sodium Na reacts with chlorine Cl, these are the reactants and the product is sodium chloride NaCl:
the common properties of such type of reactions are as follows:
- A reaction in which heat is evolved is an exothermic reaction.
- These are shown by the negative sign of the Δ
- These are spontaneous reactions.
- Combustion reactions are exothermic reactions.
Examples of the exothermic reactions
- Domestic gas combustion: The combustion of gases for domestic use, such as methane or butane, involves the chemical reaction with oxygen with the formation of carbon dioxide and water, and the release of energy. This is a typical exothermic reaction in everyday use.
- Laundry detergent: When we dissolve a little powder detergent with water in our hands we can feel slight heating.
- Rain formation: The condensation of water vapor in the form of rain expels heat.
- Combustion: When something burns, no matter how small or large, it is always an exothermic reaction.
- Concrete: When water is added to concrete, chemical reactions release heat.
You May Also interested in: