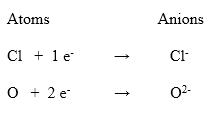Examples of Anionic molecular ions & Cationic Molecular ions
What are Cations?
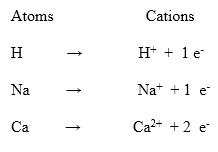
Ions is an atom or group of atoms having a charge on it. The charge may be positive or negative. There are two types of ions i.e. cations and anions. An atom or group of atoms having a positive charge on it is called a cation.
The cations are formed when atoms lose electrons from their outermost shells. for example, Na+, K+ are cations. The following equations show the formation of cations from atoms.
What are anions?
An atom or group of atoms that has a negative charge on it, is called an anion. Anion is formed by the gain or addition of electrons to an atom. for example, Cl– and O2-. The following examples show the formation of an anion by the addition of electrons to an anion.
What are molecular ions?
When a molecule loses or gains an electron, it forms a molecular ion. Hence, molecular ion or radical is a species having a positive or negative charge on it.like other ions they can be cat-ionic molecular ions ( if they carry a positive charge) or anionic molecular ions (if they carry a negative charge).
Cat-ionic molecular ions are more abundant than anionic molecular ions. For example CH4+, He+, N2, When gases are bombarded with high energy electrons in a discharge tube, they ionize to give molecular ions.
What are Free Radicals?
Free radicals are atoms or groups of atoms possessing an odd number of (unpaired) electrons. It is represented by putting a dot over the symbol of an element e.g. H. , H. ,H3C. .
Free radicals are generated by the homolytic (equal) breakage of the bond between two atoms when they absorb heat or light energy. A free radical is an extremely reactive species as it has the tendency to complete its octet.
Do you know?
Most of the universe exists in the form of plasma, the fourth state of matter. Both the cationic and anionic molecular ions are present in it.
You May Also Like: