Chemistry
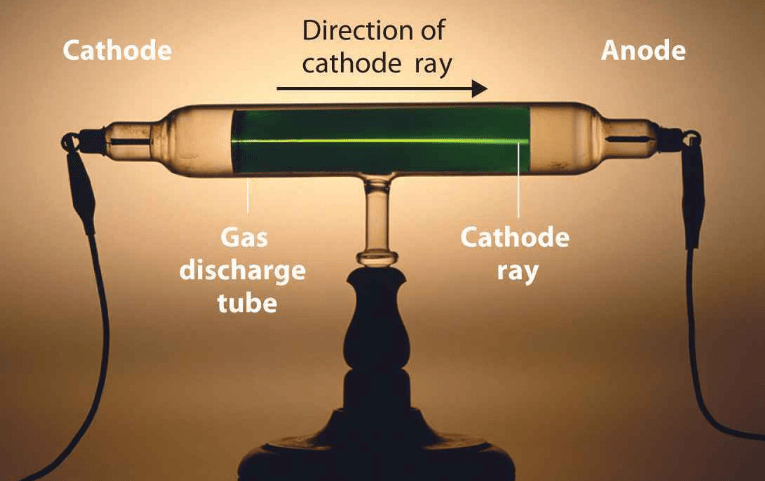
Difference between Cathode Rays and Anode Rays
Difference between Cathode Rays and Anode Rays is that Cathode Rays travel in straight lines and particles present in cathode rays are electrons while Anode Rays also travel in straight lines but particles present in anode rays are positively charged particles and charge on the particle depend upon no. of electrons lost by atoms.
Cathode rays | Anode rays |
| There are negatively charged particles. | These are positively charged particles. |
| They bend towards anode when passed through the electrical field. | They bent towards cathode when passed through the electric field. |
| The e/m ratio is the same for all gases. | The e/m value is different for all gases. |
| Emits from Cathode Rays | Emits from Anode Rays. |
| Negative Charged particles were called electrons. | Positively charged particles where called H+ Ions. |
| Originates from the Cathode. | They are produced in the space between Anode and Cathode. |
| They can produce mechanical effects. | They also can produce mechanical effects. |
You May Also Like: