Chemistry
Difference Between Rate Constant and Specific Rate Constant
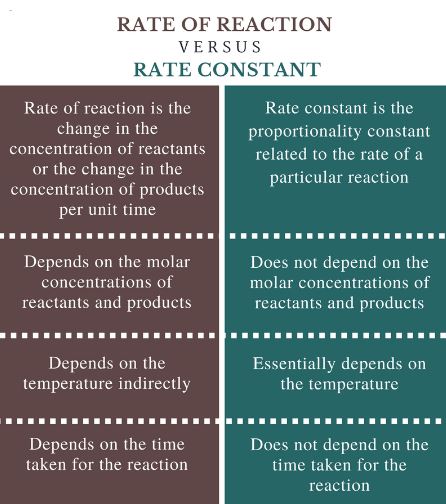
The major difference between Rate Constant and Specific Rate Constant is that Rate Constant is a constant of proportionality in the rate Law Equation and is equal to the rate of reaction when the molar concentration of each reactant is unity while Specific Rate Constant ( Rate of Reaction) is the change in concentration of the reactant or product per unit time.
Rate Constant
- It is proportionality constant in the rate expression.
- It is the ratio between the rate of reaction and the concentration of reactants.
Specific Rate Constant
- It is the rate of reaction when the molar concentration of each reactant is unity, i.e. 1 mol / dm3.
- It is equal to the rate of a reaction.