Electrical Conductance with Definition and Measurement
The reciprocal of resistance is called Electrical Conductance. The most important property of metals is their ability to conduct electricity. The delocalized electrons in metal give rise to electrical and thermal conductivity. This property is mainly due to the presence of relatively loose electrons in the outermost shell of the metal and ease of their movement in the solid.
The electrical conductance of metals in group IA and IIA general decreases from top to bottom. Metals of group IB, known as coinage metals have extraordinary high values of electrical conductance. Non-metals, on the other hand, especially of group VIA and VIIA, show. Such low electrical conductance that can be regarded as non-conductors.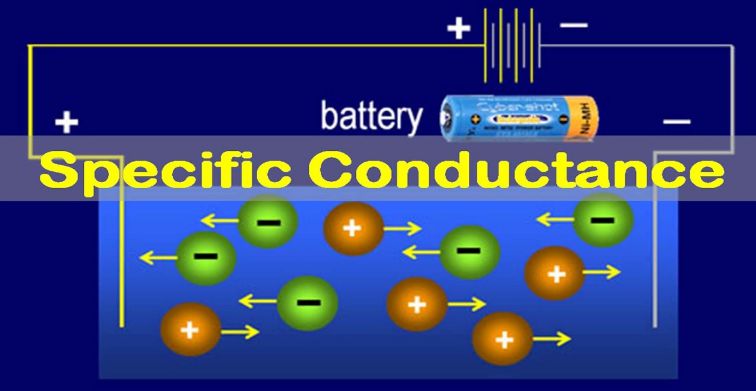
Carbon, in the form of a diamond, is a non-conductor, while in the form of graphite, it is a fairly good conductor. The lower elements of group IVA, tin, and lead, are the fairly good conductor and their values of electrical conductivity are comparable with those of their counterparts in group IA.
In the series of transition metals, the values of electrical conductivity vary so abruptly that no general trend can be assigned to them.