Relative atomic Mass and Atomic Mass unit with formula & examples
As we know that the mass of an atom is too small to be determined practically. However, certain instruments enable us to determined the ration of the atomic masses of various elements to that of carbon-12 atoms. This ratio is known as the relative atomic mass of the element.
[wp_ad_camp_1]
The relative atomic mass of an element is the average mass of the atoms of that element as compared to 1/12th (one-twelfth) the mass of an atom of carbon -12 isotope (having different mass number but same atomic number). Based on carbon -12 standard, the mass of an atom of carbon is 12 units and 1/12th of it comes to be 1 unit.
When we compare atomic masses of other elements with atomic mass of carbon-12 atom, they are expressed as relative atomic masses of those elements. The unit for relative atomic masses is called atomic mass unit, with symbol ‘amu’, one atomic mass unit is 1/12 the mass of one atom of carbon-12. When this atomic mass unit is expressed in grams, it is;
For example:
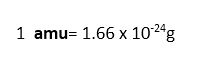

It’s really a cool and useful piece of information. I’m satisfied that you simply shared this useful information with us. Please keep us informed like this. Thank you for sharing.