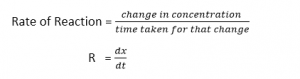Chemical Kinetics: Rate, Laws, Equations and Examples | Industrial Importance
Chemical kinetics is the branch of chemistry which deals with the study of:
- Rates of the chemical reactions.
- Factors affecting the rate of reactions.
- The mechanism through which reactions proceeds.
- The optimum condition for maximum yield of products.
[wp_ad_camp_1]
The rate of Reaction in chemical kinetics
The rate of reaction is defined as “ The quality of reactants consumed or quantity of products produced per unit time”.
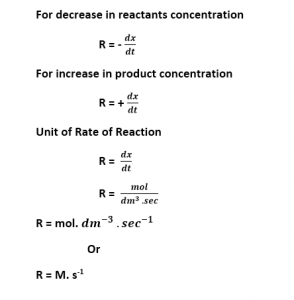
Formula
Where,
dx = small change in the concentration of reactants or products.
dt = small change in the time.
Derivation of Rate Constant and Rate Expression
Consider the following hypothetical reaction:
According to the law of mass action,
Rate of Reaction = K [A] [B]
This is the rate expression and where ‘K’ is the rate constant.
[wp_ad_camp_2]
Specific Rate Constant
When the concentration of each reactant is unity, that is, 1mol / dm3 , so the rate constant is known as ‘Specific Rate Constant’.
As we know that:
So, the rate of a reaction when the molar concentration of each reactant is unity is known as the specific rate constant.
Rate Equation OR Rate Law
An experimentally determined mathematical expression which relates the molar concentration of reactants to the reaction rate is known as rate equation or rate law.
Characteristics of Rate Constant (K)
- Its value remains constant at the constant temperature.
- Its value varies with temperature.
- Its value is independent of the concentration of reactants i.e. its value remains constant even the concentration of reactants is altered.
[wp_ad_camp_3]
Types of Reaction Based on Reaction Velocity
On the basis of their rates or velocity, there are three types of reactions which are as follows:
- Reaction proceeding at a slow rate.
- Reactions proceeding at a high rate.
- Reactions proceeding at a moderate rate.
Reaction Proceeding at a slow rates
These are the reactions, which proceed with the extremely slow speed and take longer time for their completion. It is difficult to determine experimentally the rates of such chemical reactions.
For Example
- Rusting of iron
- Formation of Diamond in Earth’s crust.
- Radioactive decay of elements.
[wp_ad_camp_4]
Reaction proceeds at a high rates
These are the reactions, which proceed with the high speed and take very short time for their completion. It is impossible to determine experimentally the rates of such chemical reactions.
Reaction Proceed At a Moderate Rates
These are the reactions, which proceed at experimentally measurable rates, i.e. with a limited (moderate) speed and completed at the most, in few hours.
Order of Reaction
“It is the sum of all the exponents of the concentration of reactants involved in the rate equation”.