Difference Between Ester And Ether
The Major Difference Between Ester And Ether is that Ester needed two carbon and oxygen atoms to complete its structure, derived from carboxylic acids, and have carbonyl group while Ether needed one oxygen and two carbon atoms to complete its structure. moreover, they do not have carbonyl groups and derived from alcohol.
Ester vs Ether in Tabular Form
| due to the carbonyl group, it can polarize Easily. | Doesn’t have a carbonyl group so it is unpolarized. |
| they are derived often from carboxylic acids | they are often derived from alcohol. |
| –COO functional group is present in Esters. | –O- functional group present in Ethers. |
| due to the. carbonyl group, they do not have symmetrical structures. | Ethers can have a symmetrical structure if both alkyl groups on either side of the oxygen atom in an ether group are similar. |
| carbon and oxygen atoms are linked with a double bond. | carbon and oxygen atom is linked with a single bond. |
| examples of ester: Ethyl propanoate | examples of ether: Dimethyl ether |
Explanation:
Ester and Ether both are functional groups and are used to categorized organic chemical compounds. ether is an organic molecule having two carbon atoms bonded with a single oxygen atom for example dimethyl ether while Ester is a Carboxylic group containing one carbon and one oxygen atom bonded together, for example, ethyl propanoate. hence, they are differed from each other due to their structure.
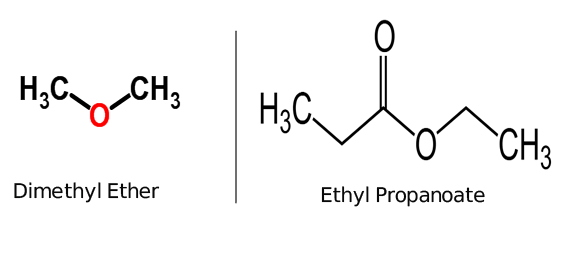 two oxygen atoms and two carbon atoms are always necessary to complete the characteristic structure of the ester group. on the other hand, one oxygen atom and two carbon atoms complete the structure of the ether group.
two oxygen atoms and two carbon atoms are always necessary to complete the characteristic structure of the ester group. on the other hand, one oxygen atom and two carbon atoms complete the structure of the ether group.
You May Also Like: