Mole (chemist secret unit): Definition, Examples and calculation Formula
A mole is defined as the amount (mass) of a substance that contains 6.02 x 1023 number of particles ( atoms, molecules, or formula units). It establishes a link between the mass of a substance and the number of particles as shown in the summary of molar calculations. It is abbreviated as ‘ mole’.
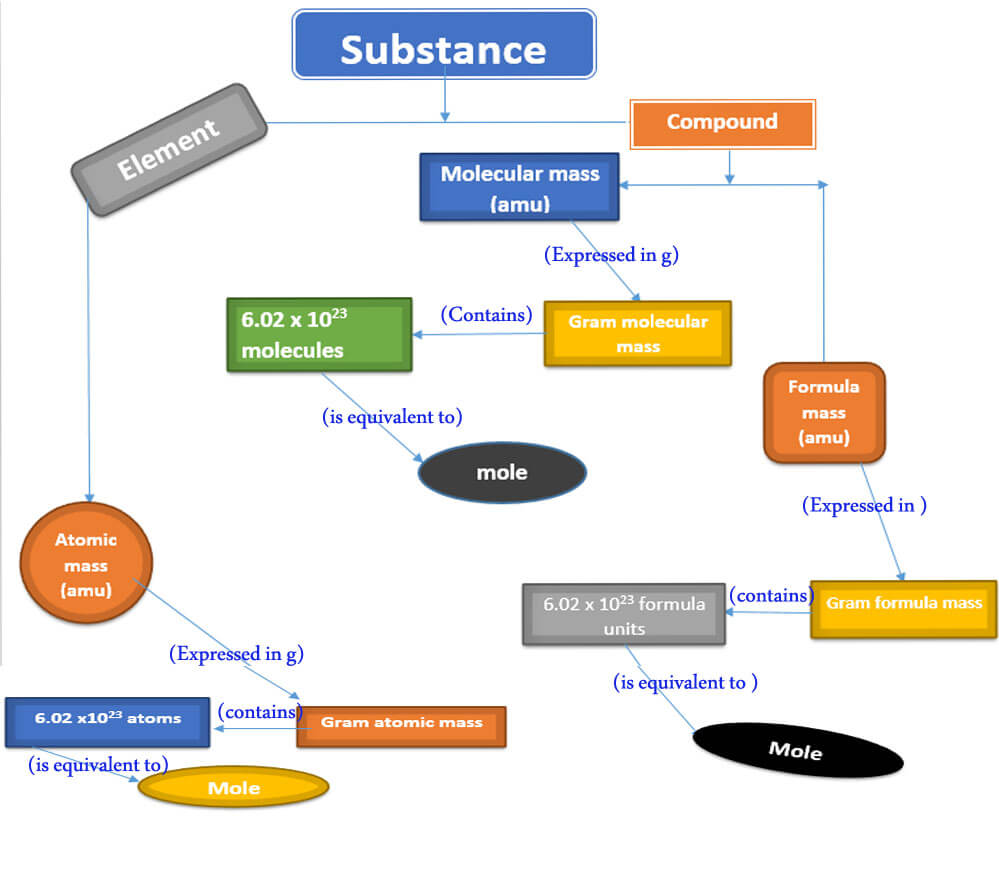
You know that a substance may be an element or compound ( molecular or ionic). Mass of the substance is either one of the following: atomic mass, molecular mass, or formula mass. These masses are expressed in the atomic mass unit (AMU). But when these masses are expressed in grams, they are called as molar masses.
Scientists have agreed that Avogadro’s number of particles are present in one molar mass of a substance. Thus, the quantitative definition can be defined as the atomic mass, molecular mass, or formula mass of a substance expressed in grams is called the mole.
For example:
The atomic mass of carbon expressed as 12g = 1 mole of carbon
The molecular mass of H2O expressed as 18g = 1 mole of water
The molecular mass of H2SO4 expressed as 98g = 1 mole of H2SO4
The formula mass of NaCl expressed as 58.5 g = 1 mole of NaCl
Thus, the relation between mole and mass can be expressed as:
A detailed relationship between a substance and through molar mass and number of particles is present here.
A summary showing a relationship between a substance and a mole.
Example of the calculation of Moles:
Calculate the gram molecule (number of moles) in 40 g of H3PO4.
Solution:
Given the mass of H3PO4 = 40 g
Molecular mass of H3PO4 = 98 gmol-1
Putting these values in the equation
Therefore, 40 grams will contain a 0.408-gram molecule (mol) of H3PO4.
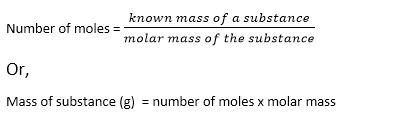
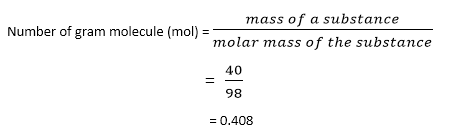

Pl can you explain history why mole is secrete unit in chemistry…