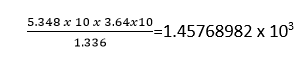Difference Between Scientific Notation and Significant figures
The difference Between Scientific Notation and Significant figures is that Scientific Notation is a method in mathematics used to express too large or small numbers into a convenient decimal form with ease and clarity. while the Significant figures are particular digits in numbers or digits. those numbers are digits only called significant figures that are part of numerical quantity.
what is Scientific Notation?
Numbers are expressed in a standard form called scientific notation, which employs the power of ten. The internationally accepted practice is that there should be only one non-zero digit left of the decimal. Thus the number 1347 should be written as 1.347 x 10-3 and .0023 should be expressed as 2.3 x 10-3.
What are Significant Figures?
in any measurement, the accurately known digits and the first doubtful digit are called significant figures. in other words, a significant figure is the one that is known to be reasonably reliable.
how to increase the number of significant figures?
we can increase the number of significant figures in measurement by improving the quality of our measuring instrument.
General Rules for deciding number of significant figures
(1) digits:
- all digits 1,2,3,4,5,6,7,8,9 are significant.
- zero may or may not be significant.
Rules for Zeros
- A zero between two significant figures is itself significant.
- zero to the left of significant figures are not significant.
for example, none of the zeros in 0.0046 or 02.59 is significant.
(note: these zeros are used only to locate decimal position)
- zeros to the right of the significant figure may or may not be significant.
- in decimal fractions, zeros to the right of the significant figures are significant.
for example, all the zeros in 3.570 or 74,000 are significant. however, in integers such as 8000 kg, the number of significant zeros is determined by the accuracy of the measuring instrument.
- if the measuring scale has a least count of 1 kg then there are four significant figures written in scientific notation as 8.000 x 103 Kg.
- if the least count of the scale is 10 kg, then the number of significant figures will be 3 written in scientific notation as 8.000 x 103 Kg.
- if the least count of the scale is 100 kg, then the number of significant figures will be 2 written in scientific notation as 8.0 x103 Kg.
- if the least count of the scale is 1000 kg, then the number of significant figures will be 1 written in scientific notation as 8 x103 Kg.
when the measurement is recorded in scientific notation or standard form, the figures other than the powers of ten are significant figures.
for example, a measurement recorded as 8.70 x104 Kg has three significant figures.
(2) Multiplication and division of numbers
in multiplying or dividing the number, keep a number of significant figures in the product or quotient not more than that contained in the least accurate factor.
as the factor 3.64 x104, the least accurate in the above calculation had three significant figures, the answer should be written to three significant figures only.
(3) Addition or Subtraction of Numbers
in addition or subtraction of numbers, the number of decimal places in the answer should be equal to the smallest number of decimal places in any of the quantities being added or subtracted.
in this case, the number of significant figures is not important. it is the position of decimal that matters.
for example, suppose we wish to add the following quantities expressed in meters.
(I) 72.1+3.42+0.003=75.523
(ii) 2.7543+4.10+1.273=8.1273
in the case of (1), 72.1 have the smallest number of decimal places, thus the answer is round off to the same position which is 75.5m.
in the case of (ii), the number 4.10 has the smallest number of decimal places, and hence, the answer is rounded off to the same decimal positions which are then 8.13m.
Do You Know?
Mass can be thought of as a form of energy. in effect, the mass is a highly concentrated form of energy. Einstein’s famous equation, E=mc2 means energy = mass x(speed of light)2. according to this equation, 1 kg mass is actually 9×1016J.
Rounding Off Data
the non-significant figures should be deleted by using the following rules:
- if the first digit dropped is less than 5, the last digit retained should remain unchanged.
- if the first digit dropped is more than 5, the digit to be retained is increased by one.
- if the digit to be dropped is 5, the previous digit which is to be retained is increased by one, if it is odd and retained as such if it is even. for example, the following numbers are rounded off to three significant figures as follows;
43.75 is rounded off as 43.8
56.8546 is rounded off as 56.9
73.650 is rounded off as 73.6
64.350 is rounded off as 64.4
You May Also Like: