Chemistry
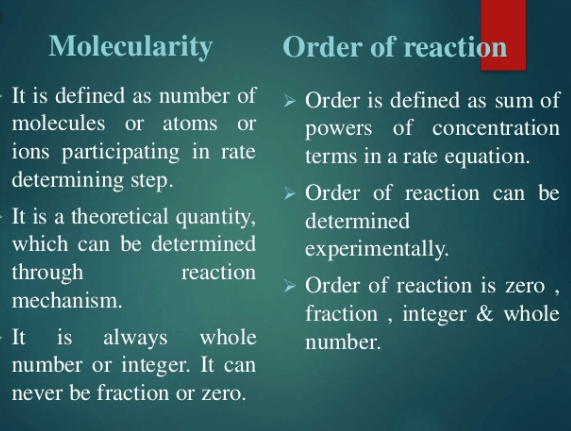
difference between molecularity and order of a reaction
The Major difference between molecularity and order of a reaction is that Molecularity is the number of atoms, ions or molecules that must collide with one another simultaneously and result in a chemical reaction while the order of a reaction is the sum of the concentrations on which the rate of reaction actually depends.
Order of Reaction
- It is the sum of all the exponents of the concentration of reactants involved in rate equation.
- Its value can be in whole number or fraction.
- Its value can never be more than three.
- It can be zero.
Molecularity of Reaction
- It is the total number of atoms, molecules or ions present in a single step.
- It’s value always in the whole number.
- Its value can be more than three.
- It can never be zero.