Chemistry
Difference Between Bonding and Antibonding Molecular Orbital
difference between Bonding molecular orbital and Anti-Bonding Molecular orbital is that Bonding Molecular orbitals formed by the addition overlap of atomic orbitals and are formed when lobe of atomic orbitals have different signs while Antibonding molecular orbitals are formed by the subtraction overlap of the atomic orbitals and are formed with same signs of atomic lobes.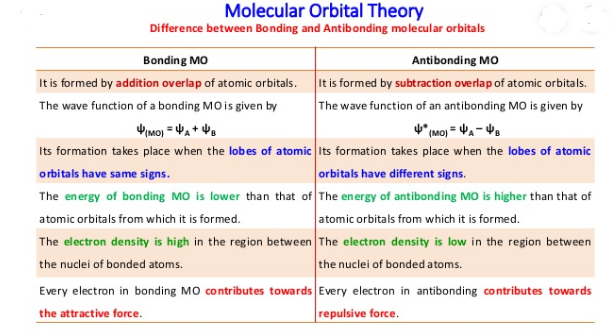
Difference Between Bonding and Antibonding Molecular Orbital
Bonding Molecular Orbital
- It has lower energy than either of a component of atomic orbitals.
- It has a higher electron density in the region between two nuclei.
- It is more stable.
- It is denoted by σ
- Electrons present in bonding MO contribute towards attraction between two nuclei.
Anti-Bonding Molecular Orbital
- It is of higher energy than the atomic orbitals.
- It has zero or low electrons probability between the regions of two nuclei.
- It is less stable.
- It is designed by σ* etc.
- Electrons present in anti-bonding MO contribute towards repulsion between two nuclei.
You May Also Like: