Preparation of Buffers and Buffer Action with Significance
Buffers: The addition of a little strong acid or base causes a change in the pH of a solution. For example, the addition of one drop of concentrated HCI to a liter of pure water (pH 7) drops the pH of the system from 7 Lo 4 and results in a 1000 fold change in acidity. If such a change occurs in a living system the results are disastrous. Certain combinations of solutes formed by mixing together a weak acid and its conjugate base can prevent large changes in pH when strong bases or acids are added to the system.
Preparation of Buffers and Buffer Action
The buffers can be prepared by mixing a weak acid with its salt. For example, by mixing acetic acid (CHCOOH) and sodium acetate (CH3COONa). One part of the buffer can neutralize protons (He) and the other part hydroxyl ion (OH). Therefore, these solutions can effectively slow down the rate of pH change.
For example, if an acid, hydrochloric acid (HCI) to an acetic acid-sodium acetate buffer solution, the sodium acetate will react with it and sodium chloride (NaCl) and acetic acid are formed. The acetic acid is a weak acid, therefore releases less H+ ions and result in a slight change in PH. Similarly, if a base, sodium hydroxide (NOH) is added t1 the buffer solution, acetic acids react with it and produce sodium acetate and water. The water ionizes very little and cannot bring profound pH change.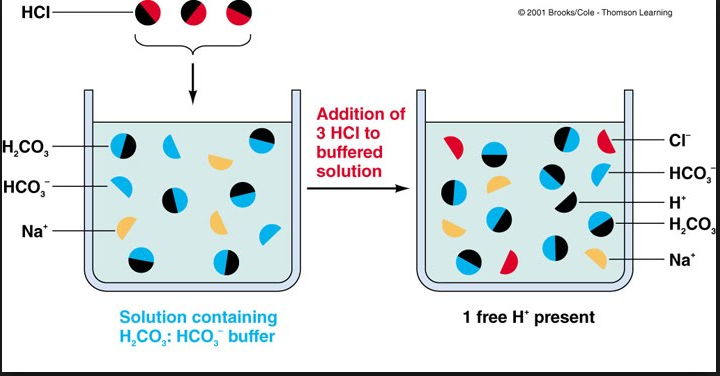
The principal buffer at work inside the cells is the phosphate buffer It consists of pairs of ions, HPO4 – and H2PO4 (the mono hydrogen and dihydrogen phosphate ions).
The dihydrogen phosphate ion (H2P04) is the acid Member of the buffer solution and will neutralize any OH on added to the system by donating a proton (H) to it. The monohydrogen phosphate ion (HPO4) is the conjugate base of the buffer solution and will neutralize any H + added to the system as it is a good proton acceptor.
H2FO4 + OH → HPO4 + H2O
HPO4 + H → H2PO4
Other buffer action mixtures are HCI and Phthalate salts, Boric acid and Sodium hydroxide, Carbonates and Borates, and Potassium dihydrogen phosphate, and Disodium phosphate.
Biological Significance of Buffers
Buffer solutions play significant roles in the normal functioning of living organisms. These are:
- Buffer solutions are abundant in living plant cells and play a vital role in their existence. Enzymes, the organic catalysts of life, generally function within a narrow pH range. A slight change in pH impairs or completely inhibits enzymatic activity. For example, a slight change in pH affects the conversion of starch to glucose by phosphorylase which is believed to be responsible for bringing turgidity in guard cells that result in the opening and closing of stomata. The phosphorylase is much sensitive to pH changes. Similarly, energy reactions especially the formation of ATP are catalyzed by enzymes and their normal working require optimum pH range.
- The buffers are used in bacteriological and fungal researches to maintain the pH of the culture medium because during reproduction bacteria produce acids that result in changes in PH. The buffers are added to these media which prevent pH change.
- Blood pH in humans must stay within the range of 7.35 and 7.45. An increase in acidity causes acidosis and a decrease in acidity result in alkalosis. The acidosis is characterized by untreated diabetes (coma) and emphysema (shallow breathing). The alkalosis results in vomiting etc. In advanced stages, acidosis and alkalosis interfere with the smooth working of PH.
The principal buffer ¡n blood ¡s the carbonate buffer. It consists of conjugate pair, carbonic acid, and bicarbonate ion (H2C03 and HC03). Carbonic add neutralize OH– and prevents alkalosis, whereas the bicarbonate neutralizes H+ proton to prevent acidosis.


I like this web blog very much, Its a very nice spot to read and find information.
Thank You!