Nitrogen Cycle | Uses & Importance of Nitrogen in Life | Nitrogen Fixation
Importance of Nitrogen Cycle
All living things need nitrogen in a form their bodies can use. It is important to plants and animals as well as to humans. It is a necessary part of the protein substance which is man’s body-building material.
Without this substance, no one could grow or repair damaged or worn-out tissues. While oxygen makes up only 21% of the air we breathe, nitrogen above a square kilometer of the Earth’s surface. It is a colorless, odorless, and tasteless gas that dissolves only slightly in water.
It would seem easy for living things to get the nitrogen they need since it is all around them in the air. But human bodies cannot use pure nitrogen. When breathed in, it dilutes the oxygen. So it is useful only when combined with other chemicals to form compounds. In nature, only a few plants, the legumes, such as beans, peas, and clover are able to use pure nitrogen from the air. But all plants can use the simple nitrogen compounds of the soil in which they grow.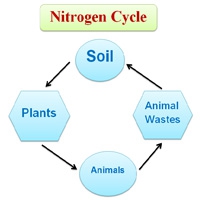
A nitrogen cycle is thus set up in nature which enables plants and animals to maintain life. Plants get simple nitrogen compounds from the soil and unite them with carbon to make proteins. Animals get the nitrogen they need by eating the plants. The nitrogen is returned to the soil as waste. Certain bacteria turn this waste back into simple nitrogen compounds and the plants are able to use it again. So the cycle is complete. The supply of nitrogen is also replenished by bacteria that take nitrogen from the air and “fix” it in the ground.
Nitrogen Cycle
Nitrogen is essential for plants and animals as it is a constituent of proteins and nucleic acids. The earth’s atmosphere contains about 78 % of the volume of nitrogen gas. Nitrate and nitrites of the soil are absorbed by the plants. Plants use nitrogenous compounds to manufacture proteins using the energy got from photosynthesis.
Plants are the major source of food for all animals. animals get their sources of food from plants. When plants and animals die, the nitrogenous compound of their bodies is decomposed and convert it into nitrates. In the case of nodules in leguminous plants, the addition of nitrogen to the soil occurs through the death of the old nodules, or by the death of roots themselves.
The nitrogenous wastes of animals and nitrogen compounds of dead organisms are decomposed by saprophytic soil bacteria and fungi to form simple substances like water amino acids. Amino acids are converted into ammonia and ammonium ions are called ammonification. Ammonia and ammonium ions are converted into nitrates by nitrifying bacteria. Nitrosomonas (bacteria) convert ammonium to nitrates and Nitrobacteria (Bacteria) convert nitrites to nitrates. The nitrates of the soil are then absorbed by plants.
Certain micro-organisms also fix nitrogen. Some of the micro-organisms live in root nodules of legumes (gram, pulses, etc.) are some life freely in the soil. These micro-organisms form amino acids. Also during thunderstorms, the nitrogen of the air combines with the oxygen to form various oxides of nitrogen. These oxides mixed with water to form nitrous acid and nitric acid which when combined with mineral salts in the soil form nitrates. These nitrates are also absorbed by plants and thus the nitrogen cycle continues.
Nitrogen is an important component of many biomolecules, like protein and nucleic acid (DNA and RNA). The atmosphere is the reservoir of free gaseous nitrogen. Living organisms cannot pick up this gaseous nitrogen directly from the atmosphere (except for nitrogen-fixing bacteria). It has to be converted into nitrates to be utilized by plants. Nitrogen cycling involves several stages:
Formation of Nitrates
It is done in the following ways:
Nitrogen Fixation
The conversion of nitrogen gas into nitrates is called nitrogen fixation. It occurs in the following ways
- Thunderstorms and lightning convert atmospheric gaseous nitrogen to oxides of nitrogen. These oxides dissolve in water and form nitrous acid and nitric acid. The acid is turned combine with the other salts to produce ‘nitrates’. It is called atmospheric nitrogen Fixation.
- Some bacteria also have the ability to transform gaseous nitrogen into nitrous. It is called biological nitrogen fixation. Some of these nitrogen-fixing bacteria live as symbionts and many are free living.
- Nitrogen fixation is also doe in industries. In industrial nitrogen fixation, hydrogen is combined with atmospheric nitrogen under high pressure and temperature. It also produces ammonia which is further converted into ammonium nitrate.
- Ammonification and Nitrification: Ammonification is the breakdown of the proteins of dead organisms and nitrogenous wastes (urea, uric acid, etc.) to ammonia. It is done by ammonifying bacteria. After the formation of ammonia, it is converted into nitrites and nitrates. It is called nitrification and is done by nitrifying bacteria. First, ammonia is converted into nitrites by bacteria (e.g. Nitrosomonas). The nitrites are then converted into nitrates by other bacteria (e.g. Nitrobacter).
- Assimilation: The nitrates formed by the above processes, are absorbed by the plants and are utilized for making proteins, etc. Animals take nitrogenous compounds from the plants. The utilization of nitrates by organisms is called assimilation.
- Denitrification: It is a biological process in which nitrates and nitrites are reduced to nitrogen gas by denitrifying bacteria. By this process, nitrogen is returned to the atmosphere.
Excessive Denitrification reduces soil fertility and is stimulated by waterlogging, lack of aeration, and accumulation of organic matter in the soil.



Thanks, it is very informative
Thanks, it’s very informative