Difference Between Positive Catalyst and Negative Catalyst
in chemistry, A Catalyst Speed up or increase/decrease the rate of a chemical reaction but not chemically charged 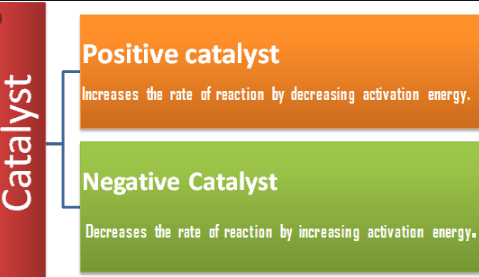 or participate in the reaction itself. the phenomenon of acceleration of a chemical reaction by a catalyst is known as catalysis. Catalysts are divided into two types: positive catalyst and Negative Catalyst. The basic difference Between positive catalyst and negative catalyst is that Those substances which increase the rate of a chemical reaction are called positive catalyst while the substances which decrease the rate of a chemical reaction is called Negative Catalyst.
or participate in the reaction itself. the phenomenon of acceleration of a chemical reaction by a catalyst is known as catalysis. Catalysts are divided into two types: positive catalyst and Negative Catalyst. The basic difference Between positive catalyst and negative catalyst is that Those substances which increase the rate of a chemical reaction are called positive catalyst while the substances which decrease the rate of a chemical reaction is called Negative Catalyst.
What is Catalysis?
in chemical chemistry, Catalysis is the process of increasing or modifying a chemical reaction or the rate of a chemical reaction by with the use of a catalyst. it only works when there is a chemical reaction present already and required in a very small amount.
What is a Positive Catalyst?
It has the ability to increase the rate of reaction by decreasing the activation energy.
- A catalyst that increases the rate of a reaction without being used.
- A decreases the Activation energy of reactant molecules.
- It is also called the Promoter.
What is a Negative Catalyst?
conversely, Negative Catalyst has the Ability to decrease the rate of reaction by increasing the activation energy.
- A catalyst that decreases the rate of a reaction without being used.
- It increases the Activation energy of the reactant molecules.
- It is also called Inhibitor.
