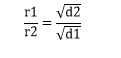Chemistry
Graham’s Law of Diffusion: Statement & Formula
Graham’s Law of Diffusion: Graham in 1829, observed the spreading out or diffusion of gases. He postulated that the rate of the diffusion of the gas does not depend upon gravity, but it depends upon the temperature of a gas. 
This law states that the “rate of diffusion of a gas is inversely proportional to the Square root of the density of the gas at a given temperature.”
If we have two gases having rates of diffusion as r1, r2, with densities d1 and d2, then
Dividing the two equations,
![]() We also know that densities are directly proportional to the molar masses,
We also know that densities are directly proportional to the molar masses,
so,
Graham’s Law Formula
It means that the rate of diffusion can be calculated from the densities of gases and molar masses of the gases.