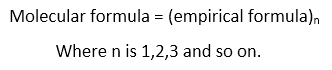Determining Empirical formula and molecular formula with examples
What is empirical formula ?
Chemical formula are of two types. The simplest type of formula is empirical’s formula. It is the simplest whole number ratio of atoms present in a compound. The empirical formula of compound is determined by knowing the percentage composition of a compound. However, here we will explain it with simple examples.
[wp_ad_camp_1]
The covalent compound silica (sand) has simplest ratio 1:2 of silicon and oxygen respectively. Therefore its empirical formula is SiO2. Similarly, glucose has simplest ratio 1:2:1 of carbon, hydrogen and oxygen respectively. Hence its empirical formulae is CH2O.
As discussed earlier, the ionic compounds exists in three dimensional network forms. Each ion is surrounded by oppositely charged ions in which a way to form electrically neutral compound. Therefore, the simplest unit taken as a representative of an ionic compound is called formula unit.
It is defined as the simplest whole number ratio of ions, as present in the ionic compound. In other words, ionic compounds have only empirical formulae. For example, formula unit of common salt consist of one Na+ and one Cl– ion and its empirical formulae is NaCl. Similarly, formula unit of potassium bromide is KBr, which is also its empirical formulae.
How to find molecular formula
[wp_ad_camp_2]
Molecules are formed by the combination of atoms. These molecules are represented by molecular formula that show actual number of atoms of each element present in a molecule of that compound. Molecular formulae is derived from empirical formula by the following relationship:
For example, molecular formulae of benzene is C6H6 which is derived from the empirical formulae CH where the value of n is 6.
The molecular formula of a compound may be same or a multiple of the empiricals formula. A few compounds having different empirical and molecular formulae are shown bellow in the table.
Some Compounds with their Empirical and Molecular Formulae
| Compound | Empirical formula | Molecular formula |
| Hydrogen peroxide | HO | H2O2 |
| Benzene | CH | C6H6 |
| Glucose | CH2O | C6H12O6 |
Some compounds may have same empirical and molecular formulae e.g water (H2O), hydrochloric acid (HCl), etc.