Difference Between Evaporation and Distillation
The method of changing liquid into a gas by applying an external source like the heat is called Evaporation and acquiring gas or vapors from the liquid surface is known as Distillation. the Major Difference Between Evaporation and Distillation is that in Evaporation vapors are not collected, concentrated products are obtained after evaporation and used for removing impurities while in Distillation vapors are condensed, the distillate is obtained after distillation and used for separation and purification.
Difference Between Evaporation and Distillation in Tabular form
| Evaporation | Distillation |
| the process of converting liquids into gases with the help of heat is called Evaporation. in this process, the molecules of the liquid quickly transform into vapors or gas. | in the distillation process, the steam or liquid vapors are produced with the help of heating liquids. |
| in this process, vapors are not collected. | in this process, vapors are condensed and collected. |
| Evaporation is a gradual and slow process. | Distillation generally a Quick and rapid process. |
| concentration product is obtained after the process done. | distillate product is obtained after the process has done. |
| it occurs at the surface of the liquid. | it doesn’t only occur at the surface of liquids. |
| it does not dais to be a separation technique. | it is a separation technique. |
| liquid bubbles do not appear on reaching the boiling point. | liquid bubbles are formed when the liquid reaches the boiling point. |
| not a separation technique. | it is a separation technique. |
| Liquid vapourization comes/happens below the boiling point. | Liquid vapourization comes/happens exactly at the boiling point. |
| a common example of simple Evaporation is Drying clothes under the sun. | a common example of simple Distillation is the purification of liquids from mixtures. |
Evaporation and Distillation Explanation:
you may already know that matter naturally exists in three fundamental states i.e., solid, liquid, and gas. so, there is a possibility that we can convert or interchange one state of matter into others. and this process is called phase change of matter. this phase change sometimes occurs due to evaporation and sometimes due to distillation.
the primary difference between evaporation and distillation is that evaporation is a change in the state of matter. for example, ice converts into the water called evaporation. while distillation is a separation process. both processes have their own significance in nature and used for different purposes. distillation is initiate by some external source while the other is started by a naturally occurring source.
they are inter-connected in some manners that evaporation can happen within distillation while distillation needs heat or temperature to start. so, it cannot happen in evaporation.
in evaporation, liquids are changed into a gaseous state. there are numerous factors that affect the process of evaporation like temperature, surface area, density, and concentration of different substances and mixtures, etc.
distillation is a physical process that separates different substances and mixtures from each other. this strongly relies on the boiling points of mixtures. these mixtures or substances are separated by distillation on different boiling points or on being heated. in this way, the evaporation takes place totally within distillation.
What is evaporation?
if we supply energy to different molecules of substances, they turn into vapors. the following process is called  evaporation. it only occurs at the surface of liquids. evaporation only occurs when the external body temperature is greater than the internal temperature of the body or substance. it refers to changing a liquid into a gaseous form by applying any source of heat to the liquid. boiling of water is a common example of evaporation.
evaporation. it only occurs at the surface of liquids. evaporation only occurs when the external body temperature is greater than the internal temperature of the body or substance. it refers to changing a liquid into a gaseous form by applying any source of heat to the liquid. boiling of water is a common example of evaporation.
Examples of Evaporation
there are several examples of evaporation that are there in nature such as the rain cycle and sweating of human bodies. due to the internal energies of bodies, sweat gets this energy and evaporates outside the body. on the other hand, the rain cycle is another perfect example. Water evaporates from the earth’s surface into the atmosphere, where it travels into the atmosphere.
Due to the cold temperature, the vapors are reduced again in water droplets, which accumulate as clouds. When the clouds are full, the raindrops fall on the ground like rain.
what is Distillation?
as we have concluded above, evaporation is a natural process, but distillation is a self-made or hand made process. it 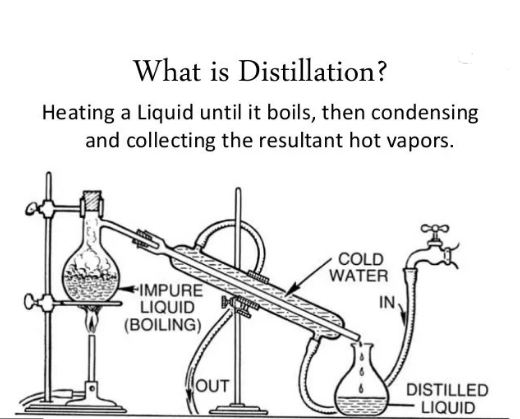 is used to separate the purest form of liquids from mixtures or other unpure liquids and substances.it always varies upon the different boiling points. because the intermolecular forces are different in different liquids, hence, distillation also varies.
is used to separate the purest form of liquids from mixtures or other unpure liquids and substances.it always varies upon the different boiling points. because the intermolecular forces are different in different liquids, hence, distillation also varies.
in other words, having different boiling points, different amounts of heat energy is required to break the bonds or intermolecular forces. it is a controlled process often used to separate different liquid mixtures. when liquids are fully boiled, two things happen to liquid molecules. they gained more vapor pressure and energy from atmospheric pressure. due to this, gas bubbles rise up and escaped from the surface.
Examples of Distillation
distillation is useful for many commercial purposes as well as chemistry labs. here are some examples:
- through this process, salted water is purified into freshwater for household uses.
- a useful process in alcoholic beverages.
- in petroleum industries, fuels and gasoline are separated from impurities or crude oil through distillation.
- air is separated into its components such as nitrogen, oxygen, and argon, etc for industrial and commercial uses.
- in chemical synthesis, no. of crude oil liquid products are separated and distilled from impurities through distillation.
You May Also Like: