What are Carbohydrates | Definition Sources & Importance
Types of Carbohydrates: Polyhydroxy aldehydes or ketones, or complex substances which yield Polyhydroxy aldehyde or ketone subunit on hydrolysis are called “Carbohydrates”.
Hydrolysis means the breakdown of large molecules into small molecules by using water. Carbohydrates are composed of carbon, hydrogen, and oxygen. The word carbohydrates literally means, “Hydrated Carbons”. The ration of hydrogen and oxygen is the same, as in water, i.e. 2:1. The number of carbon may be from three to many thousand.
Carbohydrates occur abundantly in living organisms. They are found in an organism and in almost all parts of the cell. The cellulose of wood, cotton, and paper, starches present in cereals, root tubers, cane sugar, and milk are all examples of carbohydrates. Carbohydrates play both structural and functional roles. Simple carbohydrates are the main source of energy in cells. Some carbohydrates are the main constituents of cell walls in plants and micro-organisms.
Carbohydrates in cells combine with proteins and lipids and the resultant compounds are called glycoproteins, respectively. Glycoproteins and glycolipids have a structural role in the extracellular matrix of animals and bacterial cell walls. Both these conjugated molecules are components of biological membranes.
Classification or Types of Carbohydrates
Carbohydrates are also called ‘saccharides’. The word saccharide is derived from the Greek word ‘saccharin’. It means sugar. Saccharide is taken as a unit (monomer) of carbohydrates. Carbohydrates are classified into three groups:
- Mono Saccharides (carbohydrates containing only one sugar)
- Oligo Saccharides (carbohydrates containing 3 to 10 sugars. For Example Dextrin)
- Polysaccharides (carbohydrates containing more than 10 sugars)
- Disaccharides: They consist of two molecules of monosaccharides e.g. Sucrose, Lactose, and Maltose, etc.
a) Monosaccharides
They show the following characters:
- They are simple sugars.
- They are sweet in taste.
- They are easily soluble in water. They cannot be hydrolyzed into simple sugars.
- Chemically, they may be Polyhydroxy aldehydes or ketones.
- Except for one carbon, all other carbon atoms in monosaccharides have hydroxyl groups (OH). The carbon without the OH group forms aldehyde or ketone
- The sugar with aldehyde groups is called Aldo-sugar and the sugar with ketone groups is called keto-sugars.
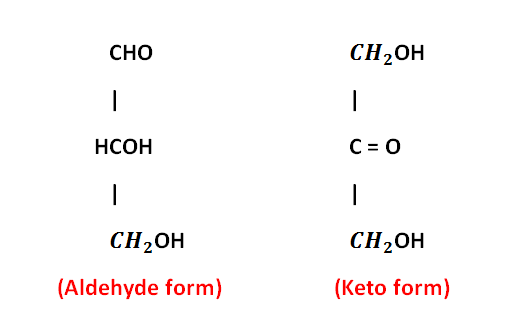
Types of Monosaccharides
Monosaccharides with 3 to 7 carbon atoms are found in nature. They are called trioses (3C), tetroses (4C), hexoses (6C), and heptoses (7C). They have a general formula. There are following types of monosaccharides:
a) Trioses:
They have three carbons. Two trioses are aldehydes (glyceraldehydes) and ketones (dihydroxyacetone). These are produced as intermediate compounds during respiration and photosynthesis.
b) Tetroses:
They have four carbons. Tetroses are rare in nature. They occur in some bacteria.
c) Pentoses:
They have five carbons. Ribose Sugar is a common pentose. It is an Aldo sugar. It gives five cornered ring structures in solution form. This ring is called a ribofuranose. It is present in ribonucleic acid RNA. Deoxyribose is also a pentose sugar. It is found in DNA.
d) Hexoses:
They have six carbons. For example, Glucose.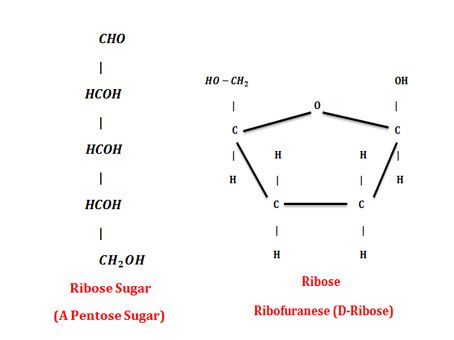
Glucose
Glucose is a hexose sugar. It is the most important biological compound. It is an Aldo sugar. It forms a six-cornered ring in solution. This ring is called the glucopyranoses.
Role of Glucose in Photosynthesis and Respiration
Glucose is naturally produced in green plants. They take from the air and water from the soil to synthesize glucose.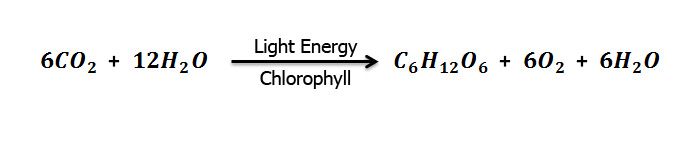
Energy is consumed in this process, this energy is provided by sunlight. This process is called photosynthesis. 717.6 Kcal of solar energy is required for the synthesis of 10g of glucose. The energy is stored in glucose as chemical energy. During oxidation (respiration) of glucose, this energy is released in the body of the organisms and used in different activities.
Importance of Glucose
Glucose has great biological importance.
- Glucose is present in a free state in fruits like grapes, figs, and dates.
- Our body normally contains 0.08% glucose.
- It is present in disaccharides and polysaccharides in combined form.
- Starch, cellulose, and glycogen give glucose on hydrolysis.
- It is a chief source of energy.
b) Oligosaccharides
Oligosaccharides show the following characteristics:
- These are less sweet in taste.
- These are less soluble in water.
- They yield 2 to 10 monosaccharides on hydrolysis.
Types of Oligosaccharides
The covalent bond formed between two monosaccharides is called Glycosidic Bond. The oligosaccharides may be:
Disaccharides:
The oligosaccharides which give two monosaccharides are called disaccharides.
Trisaccharides:
The oligosaccharides which give three monosaccharides are called Trisaccharides and so on.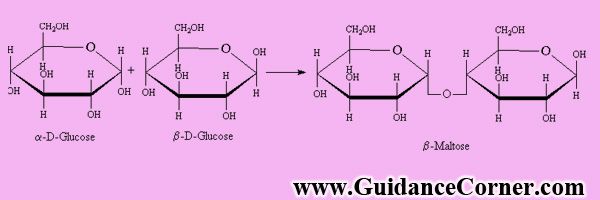
Importance of Oligosaccharides
Maltose, Sucrose, and Lactose are physiologically important disaccharides. The most common disaccharide is sucrose (Sugar Cane). Sucrose gives glucose and fructose on hydrolysis. These two are reducing (release H or absorb Oxygen) sugars. The molecular formula of sucrose is.
c) Polysaccharides
Polysaccharides show the following characteristics:
- They are tasteless.
- They are sparingly soluble in water.
- They are the most complex carbohydrates.
- They are most abundant in nature.
- They are usually branched or unbranched.
- Several monosaccharides linked by glycosidic linkage and from polysaccharides.
- They have a high molecular weight.
Examples of Polysaccharides
Some biologically important polysaccharides are starch, glycogen, cellulose, dextrin, agar, pectin, and chitin.
Starch
- It is found in fruits, grains, seeds, and tubers.
- It is the main source of carbohydrates for animals.
- It gives many molecules of glucose on hydrolysis.
- Starch gives a blue color with iodine.
- Starch has two types:
- Amylose starch: Amylose has an unbranched chain of glucose. It is soluble in hot water.
- Amylopectin: It has branched chains. It is insoluble in hot or cold water.
Glycogen
- It is also called Animal Starch. It is a chief storage compound of animals.
- It is found in the liver and muscles. It is also found in all animal cells.
- It is insoluble in water.
- It gives red in colour with iodine.
- It gives glucose on hydrolysis.
Cellulose
- It is the most abundant carbohydrate in nature.
- Cotton is a pure form of cellulose.
- It is the main constituents of the cell walls of plants.
- It is highly soluble in water.
- It yields glucose molecules on hydrolysis.
- The human digestive tract cannot digest glycogen. It is digested in herbivores due to micro-organisms (bacteria, yeasts, protozoa). These micro-organisms are present in their digestive tract of the herbivores. These micro-organisms secrete an enzyme, called cellulose. Cellulose digests the starch.
- Cellulose gives no color with iodine.
Sources of Carbohydrates
Sources of Carbohydrates are green plants. Carbohydrates are primary products of photosynthesis. Other components of plants are produced from carbohydrates by different chemical changes.
Importance or Functions of Carbohydrates
Carbohydrates occur abundantly in living organisms. They have the following importance:
- They are found in all organisms. They are present in all parts of the cells.
- They form different structures, like cellulose of wood, cotton, and papers.
- They act as storage compounds like starch and Starch is present in cereals and root tubers (Potatoes). Carbohydrates are also present in cane sugar and milk sugar.
- They are the main source of energy in the cell.
- Some carbohydrates form the cell wall in plants and micro-organisms.
- Carbohydrates combine with proteins and lipids to form glycoproteins and glycolipids. These are called conjugate compounds. These compounds play a role in the extracellular matrix of animal and bacterial cells. The conjugate compounds also have a structural role in the biological membranes.


My brother recommended I might like this web site. He was entirely right. This post actually made my day. You can not imagine just how much time I had spent for this information! Thanks!
Hello there, You’ve done an incredible job. I will certainly digg it and personally suggest to my friends. I’m confident they will be benefited from this web site.
Hello colleagues, how is the whole thing,
and what you desire to say on the topic of this
article, in my view its truly remarkable in support of me.