lipids are The heterogeneous group of compounds related to fatty acids which are insoluble in water but soluble in organic solvents, like alcohol, ether, chloroform, and benzene. Lipids include fats, oils, waxes, cholesterol, and related compounds. Fats, oils, and steroids constitute lipids. These compounds are composed of hydrogen, oxygen, and carbon, but oxygen is present in less quantity. Lipids are insoluble and less dense in water.
Lipids also consist of waxes, natural rubber, vitamin A, E & K.
Bloor in 1943 proposed the term lipid. The lipids can be defined as the group of organic compounds, which are insoluble in water and soluble in Bloor’s reagents mixture of diethyl ether and ethyl alcohol (2:1 ratio) or other fat solutions like benzene, acetone, chloroform, and ether. The lipids from fatty acids on hydrolysis.
Lipids are found both in plants and animals. In plants, they are usually present in seeds, fruits, and nuts.
Importance of Lipid Functions
Lipids perform the following functions:
- Lipids are hydrophobic compounds. Therefore, they form components of the cell membrane.
- Lipids act as storage compounds like fats.
- They have a high proportion of C-H bonds and a low proportion of oxygen. Thus lipids store the double amount of energy as compared to carbohydrates.
- Some lipids provide insulation against atmospheric heat and cold. They also act as a waterproof material. For example, waxes in the exoskeleton of insects. A wax cutin forms an additional protective layer on the cuticle of the epidermis of some plant organs like leaves, fruits, seeds, etc.
- Some lipids like steroid hormones, cholesterol perform important functions in the bodies of animals.
Classification of Lipids
Lipids are divided into the following groups:
- Acyl-Glycerol (Fats and Oils)
- Waxes
- Phospholipids
- Terpenoids
Acyl-Glycerol (Fats and oils)
The ester of fatty acids and alcohols are called Acylglycerols. An ester is a compound produced as a result of a chemical reaction of an alcohol with an acid. A water molecule is released during this reaction. The most abundant acylglycerol is triacylglycerol. It is also called triglycerides or neutral lipids. It contains three fatty acids.
OH– is released from alcohol and H+ from a fatty acid. The H and OH combine to form a water molecule. Acylglycerols form fats in plants and animals.
Alcohol
The alcohol in acylglycerols is glycerol. Glycerol is trihydroxy alcohol.
Fatty Acids
“The carboxylic acids with an even number (4-30) of carbon atoms in a straight chain with attached hydrogen and having an acidic group COOH (carboxylic group) are called fatty acids.”
Fatty acids are one of the most important components of triglycerides. Fatty acids are of two types:
Saturated Fatty Acids:
They contain no double bond, e.g. Palmitic Acid, Butyric Acid, etc.
Unsaturated Fatty Acids:
They contain up to six double bonds, e.g. Oleic Acid.
Fatty acids are straight chains in animals. While, in Plants, fatty acids are branched or ringed. Solubility of fatty acids in organic solvent and their melting point increase with the increase in a number of carbon atoms in a chain. Palmitic Acid is more soluble in the organic solvent than Butyric Acid. The melting point of Palmitic Acid is 63.1oC, while Butyric Acid is only -8oC.
Animal and Plant fats (Acylglycerol):
Fats and oils are lighter than water and have a specific gravity of about 0.8. There are followingrerences between animals and plant fats.
Plant Fats (Oils): plant Fats are usually liquid at room temperature and are called oils. They contain unsaturated fatty acids.
Animal Fats (Butter): Animal Fats are solid at room temperature. They contain saturated fatty acids.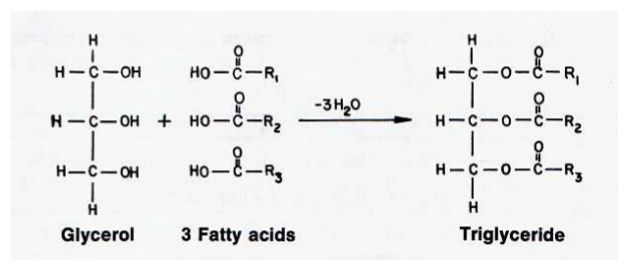
WAXES
The mixture of alkanes (with numbers of carbon from C25 to C35), alcohols, ketones, and esters of long chain fatty acids are called waxes, e.g. cutin. They perform different functions in plants and animals:
- In plants: they form a protective coating on fruits and leaves. Waxes protect plants from water loss and abrasive damage.
- In animals: some insects secrete waxes. They also provide water barrier for insects, birds, and sheep.
Waxes are the esters of fatty acids with monohydroxy alcohol of long chain or cholesterol instead of alcohol. They are found in the coating of stem, stalk, leaf fruit, and can be used in cosmetics, creams, ointment, and polishes.
Waxes are also used as machine lubricants. In the early periods, wax was obtained from whales. But, now it is obtained from a desert plant, called Simmondsia Chinensis OR Jojoba. It is rich with wax ester as storage lipids in its seeds. CH3 (CH2)4 COO (CH2)29 CH3.
Phospholipids
The lipids which are derivative of phosphatidic acid are called phospholipids. Phosphatidic acid is composed of Glycerol, fatty acids and phosphoric acid. Nitrogen bases like choline, ethanolamine and serine are important component of phospholipids. Phospholipids are present in the bacterial, animal, and plant cells. They form a part of cell membrane. Phosphatidylcholine is the most common phospholipids of cell membrane.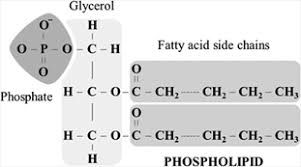
They help in regulation of cell permeability and transport process. Phospholipids are actually compound lipids, because phosphate group replaces one fatty acid of lipid. Some other compound lipids are glycolipids and sphingolipids.
Glycolipids: These lipids are linked to a sugar molecule. On hydrolysis they give sugar, fatty acid and a nitrogenous compound. They are found in chloroplast.
Spingolipids: They are found in animal and not in plants.
Terpenoids
The lipids made up of the simple repeating units of isoprenoid are called terpenoids. Different types of compounds are formed by the condensation of this unit. Terpenoids are very large and important compounds. For example, rubber, carotenoids, steroids, terpenes etc.
They are the larger lipid compounds consist of isoprenoid units (C5H8 Units). The important classes of terpenoids are:
- Terpenes
- Steroids
- Carotenoids
A. Terpenes
The group of lipids based only on isoprenoid unit 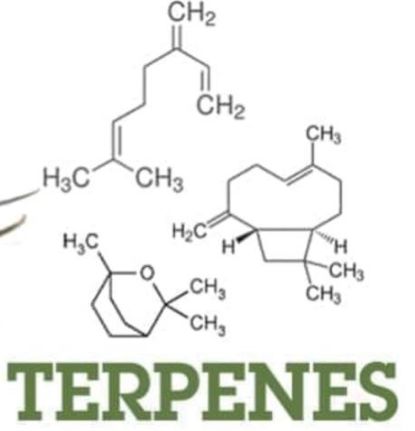 (C5H8). Terpene is the large important class of the lipids obtained from the steam distillation of plant.
(C5H8). Terpene is the large important class of the lipids obtained from the steam distillation of plant.
Some of these terpenes are essential oils responsible for odour of many plants, such as roses, peppermint, camphor, cloves, sandalwood, eucalyptus, citrus, ocimum basilicum (Niazbo) etc.
Importance of Terpenes:
- These are used as perfumes, such as from rose ceraniol is obtained, Limonine is obtained from lemon and menthol is obtained as peppermint.
- Derivatives of some terpenes are present in vitamin A1 and A2.
- They are important part of Chlorophyll.
- These are intermediate compounds during cholesterol formation.
- These are used in the synthesis of rubber and latex.
B. Steroids
These are the fats, which do not form soap with NaOH or KOH. They consist of three six membered rings A, B and C and one five-membered ring. This structure is called perhydro-cyclopentano-phenanthrene. These rings are attached together with total 17 carbon atoms, called Steroid Nucleus.
The common examples of steroidal lipids are cholesterol, vitamin D and cortisone. Cholesterol is present in animal tissues, nerve tissues and spinal cord. It also occurs in the egg yolk, various oils and fats. It is formed in liver. Steroids acts as a precursor in the biosynthesis of sex-hormones, vitamins and steroids hormones, e.g. cortisone.
C. Carotenoids
Carotenoids are also included in this group. These are the pigments producing red, orange, yellow and brown colours in plants. Vitamin A is derivative of carotene, which is found in carrots, tomatoes, and leaves etc. The red pigment carotene and yellow pigment xanthophylls are present in many plants. Tetrapyrrole pigment is present in chlorophyll and cytochromes.