Ionization Energy: Formula & Trends
The process of removing an electron from an isolated atom to form a positive ion is called Ionization. Note that ionization is an endothermic process, i.e. it requires energy to remove an electron from the attractive force of the nucleus. The ionization energy of an element is defined as the minimum amount of energy required to remove the most loosely bound electron from an isolated gaseous atom to form a cation.
For example,
Ca +(g) → Ca +(g) + e– ∇H = 590kJ/mol
Since more than one electron may be removed from an atom, one after the other. Thus, the energy required to remove an electron from an atom is often called the first ionization energy.
What is the first ionization energy?
First ionization energy is the amount of energy required to remove the first electron from the gaseous mono positive cation to form a dipositive cation. For example.
Ca+(g) → Ca2+(g) +e– ∇H=1145kJ/mol
Higher ionization energies can be defined in the same way. Ionization energies are sometimes called Ionization Potentials. Ionization energies are usually expressed in electron volts (eV) per atom or in kilojoules per mole (kJ/mol).
1eV / atom = 96.48 kJ/mol
Each electron in an atom has its own ionization energy value. The value of this energy will increase with each removed electron. Since the attractive influence of the nucleus increases and will require more energy for the removal of an electron from more positive charges. Therefore, the values of ionization energies increase from first to second to third, and so on.
Ionization energies measure how lightly electrons are bound to atoms. Low ionization energies indicate the ease of removal of electrons and hence ease of positive formation.
Factors affecting the magnitude of Ionization Potential:
The following factors influence the magnitude of the ionization potential of atoms of elements.
- Effective nuclear charge
- Atomic size i.e. atomic radius
- Principal quantum number
- Shielding effect
- Half filled and completely filled orbitals
- Nature of orbitals
- The extent of penetration of valence electrons.
Effective nuclear charge
Greater is the magnitude of effective nuclear charge, greater is the electrostatic force of attraction exerted by the nucleus on the outer electrons. Thus, it would be more difficult to remove the outermost shell electron from an atom with a higher effective nuclear charge.
This means that the greater the magnitude of effective nuclear charge, the higher is the amount of energy needed to remove the outermost shell electron. Thus, with the increase of the magnitude of effective nuclear charge, the magnitude of ionization potential also increases. The effective nuclear charge increases from left to right in a period.
Atomic size (Atomic radius)
Greater is the atomic size of an atom, farther is the outer-most shell electron from the nucleus. Hence, lesser will be the force of attraction exerted by the nucleus on the outermost shell electron.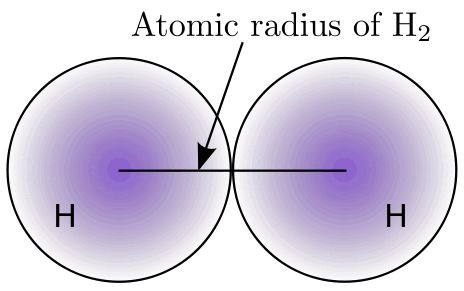
Thus with the increase of atomic size (i.e. atomic radius), it would be easier and easier to remove an electron from the outermost shell, i.e. higher is the value of the atomic radius of an atom, lower will be the ionization energy.
On proceeding from top to bottom in a group, the value of the atomic radius increases. Hence, the magnitude of ionization potential decreases. On moving from left to right in a period the value of atomic radius decreases and hence the magnitude of ionization potential increases.
Principal quantum number (n):
Greater is the value of n for the valence shell electron of an atom. Farther away, this electron will be from the nucleus, and hence lesser will be the force of attraction exerted by the nucleus on it. It means that a lesser amount of energy will be required to remove the valence shell electron. Thus, with the increase of the value of n of the valence shell electron, ionization potential decreases, and vice versa.
Thus, with the increase of the principal quantum number (n) of the orbital from which the electron is to be removed; the magnitude of the ionization potential decreases down the group.
Shielding Effect
The magnitude of the shielding effect determines the magnitude of the force of attraction between the nucleus and the valence-shell electron. Greater is the magnitude of shielding effect working on the valence-shell, smaller is the magnitude of the force of attraction between the nucleus and the valence-shell electron. Hence, lower is the amount of energy required to remove the valence-shell electron.
Thus, with the increase of the shielding effect, ionization potential decreases. As we move down a group, the number of intervening electrons increases. Hence, the magnitude of the shielding effect caused by these electrons on the valence-shell electron increases.