Hydrogen Bonding Definition and Examples in chemistry with Behavior
The electrostatic force of attraction between partially positive hydrogen atoms bonded to a high electronegativity atom ( O, N, F) and partially negative neighbouring electronegativity atom of the other molecule is termed as Hydrogen Bonding. It is also known as a protonic bridge. It is represented by dotted line ( ………..).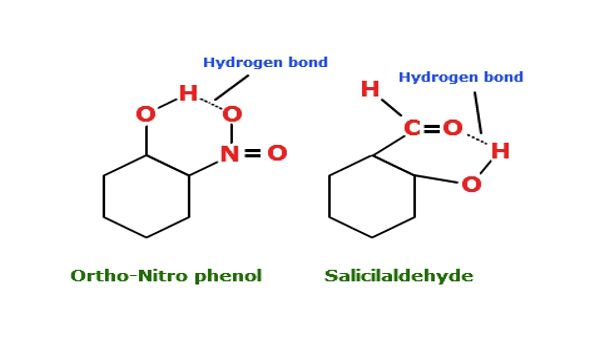
The tendency of a molecule to form hydrogen bond depends upon its ionic character, which is, in turn, depends upon the difference of electronegativities. Fluorine with the highest electronegativity forms the strongest Hydrogen Bond.
[wp_ad_camp_1]
The hydrogen bonding is the strongest of the secondary bonds, but it is much weak than a covalent bond.
The hydrogen bonding greatly affects the physical properties of a compound. Due to this bond the melting point, the boiling point, density and viscosity of a compound are increased to a great extent.
The most interesting impact of hydrogen bonding is seen in the crystalline structure of ice which causes water to behave abnormally from 0˚C to 4 ˚C (anomalous behaviour).
The density of ice is less than liquid water. When ice melts some of the hydrogen bonds are broken and the molecules pack more closely together so that the water has a high density.