Dipole Moment Factor Affecting Formula and Examples
What is the Dipole Moment?
“The extent of the tendency of a molecule for orientation in the electric field is known as dipole moment. It is represented by ‘μ’ (mu).
Mathematical derivation
Mathematically, it is measured by the magnitude of charge at each pole multiplied by the distance between two charges: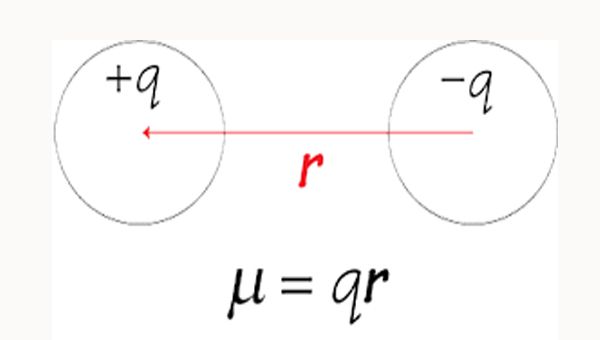
μ = e × d
Where,
μ= dipole moment
e= magnitude of the charge
d= distance between charge
Dipole Moment Unit
It was expressed in Debye units, but now they have expressed in S.I units as coulomb meter (Cm).
1 debye = 10-18 esu x cm = 3.335 x 10-30 C.m
A polar covalent bond carries partial positive and partial negative charges at opposite ends of a molecule. Such a molecule is said to be ‘dipole’ due to having opposite charges at either end. A dipole behaves like a small magnet and it tends to become oriented like a magnet in magnetic and electric fields.
Orientation in the magnetic field is the measure of the polarity of a molecule and is referred to as a dipole moment.
Factors Affecting on Dipole Moment
The dipole moment of the molecule depends on the following factors:
Polarity of Molecule
Diatomic molecule depends upon its polarity which, in turn, depends upon the difference of electronegativity. A covalent molecule consisting of identical atoms in non-polar and has dipole moment zero. But a diatomic molecule consisting of dissimilar atoms μ > 0 and it is polar.
Symmetry of Molecule
An asymmetric molecule has zero dipole moment in the polar molecule. For example, in CCl4 molecule which contains C-Cl bonds which are the polar moment. Chloroform molecule (CHCl3) on the other hand has a dipole moment due to its asymmetrical structure.
Geometry of Molecule
For polymeric molecules, dipole moment not only depends upon the polarity of its bonds but it also depends upon the geometry of the molecule. For example, SO2 and CS2 both contain polar bonds but the angular structure of SO2 results in the dipole moment of 5.336 x 10-30 C.m, while CS2 is linear μ = 0 therefore non-polar. Similarly, H2O is polar due to its angular structure having a bond angle of 104.5˚.