Difference Between Endpoint and Equivalence Point
the Major Difference Between Endpoint and Equivalence Point is that Endpoint is an estimate of the equivalence 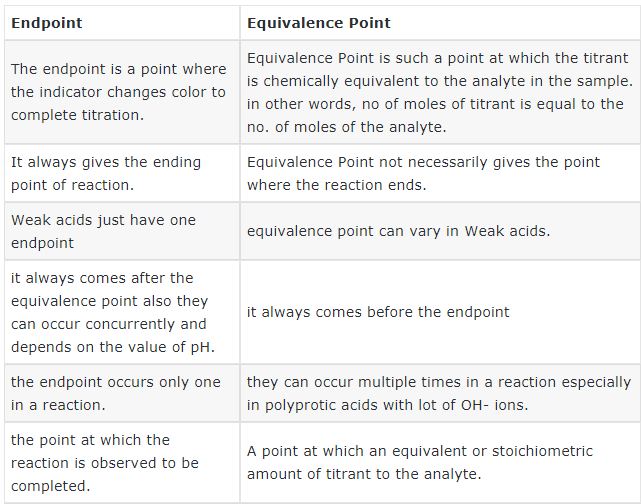 point that is observed by some physical change associated with conditions of the equivalence point while Equivalence Point is an amount of added titrant is the exact amount necessary for the stoichiometric reaction.
point that is observed by some physical change associated with conditions of the equivalence point while Equivalence Point is an amount of added titrant is the exact amount necessary for the stoichiometric reaction.
Endpoints and equivalent points are often used in titration and are refers to analytical chemistry and used to know unknown solutions in a mixture by adding known solution concentrations. they are used to determine different terms like reductants, oxidants, acids, bases, and other likewise species.
in titration, known concentrations are called titrant whereas, unknown solution concentrations are known as analyte. Titration is found mostly in redox reactions and acid-base reactions.
During these titration reactions two very important stages take place, one is the endpoint and the other is the equivalent point.
so,
What is an Equivalent Point?
when the added titrant is chemically equal or equivalent to the sample analyte, this stage is called an equivalent point. For example, in complex titration, when Aerocom Black Tea is used as a symbol, the colors at the end change from red wine to blue.
What is an Endpoint?
An endpoint is a point where the symbol of color changes appear to indicate a complete titration. Similar concepts are also known as established stoichiometric points.
During the negotiation phase, the portion of the chemical that was allowed to be added is equal to what the defendant examines in this model is called the equivalent space.
Difference Between Endpoint and Equivalence Point in Tabular Form
| Endpoint | Equivalence Point |
| The endpoint is a point where the indicator changes color to complete titration. | Equivalence Point is such a point at which the titrant is chemically equivalent to the analyte in the sample. in other words, no of moles of titrant is equal to the no. of moles of the analyte. |
| It always gives the ending point of reaction. | Equivalence Point not necessarily gives the point where the reaction ends. |
| Weak acids just have one endpoint | equivalence point can vary in Weak acids. |
| it always comes after the equivalence point also they can occur concurrently and depends on the value of pH. | it always comes before the endpoint |
| the endpoint occurs only once in a reaction. | they can occur multiple times in a reaction especially in polyprotic acids with a lot of OH- ions. |
| the point at which the reaction is observed to be completed. | A point at which an equivalent or stoichiometric amount of titrant to the analyte. |
| the selected indicator should change color very near to the equivalent point. | theoretically, at the equivalence, we can calculate the amount of titrant that is required to react exactly with the amount of analyte present. |
Note: the volume between the endpoint and equivalence point must be very small. if lots of difference appears, it is called the error in titration.
You May Also Like:




Thanks for sharing important details like this. I enjoyed reading your article, and love to know the latest updates.