What is the Difference Between Elements And Atoms?
the major Difference Between Elements And Atoms is that atoms are the smallest units that made up all matter, while elements are the substances that are made up entirely of one type of atoms. individual atoms can have an equal or different number of protons and neutrons. there are only 118 types of elements in this universe.
In this post, you are going to learn about the Elements Vs Atoms step by step with Diagram.
- an overview.
- Comparison of Table
- Questions about Atoms and Elements
- Lots more
So if you want to get benefits from this post you’ll love this post.
Let’s Dive right in..
An Overview
in many sciences, chemistry is quite interesting subject which gives us a variety of things. it gives the clue that how matter consists of different things. as a chemistry student, you’ll listen to some common terms like atoms, molecules, elements, ions, compounds, etc. the chemical substances are made up of atoms and molecules. Many of us consider atoms and elements the same but they aren’t. so what’s the difference between atoms and elements?
well, we are here to find out the answer to this question in detail.
What are the elements?
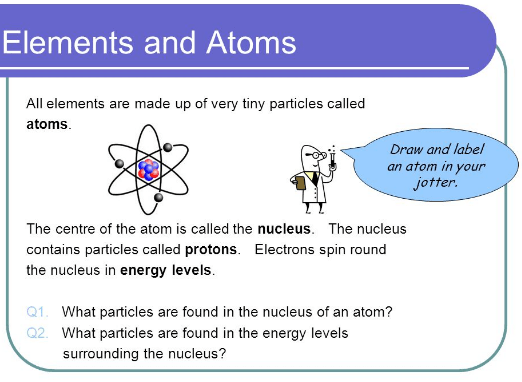
the elements in chemistry, consist of different atoms and are pure substances that cannot be broken down into pieces by some chemical methods. an element has an identical number of protons and these protons are present in the nucleus and are considered the defining property of any element. the proton number of elements is also called the atomic number of that element. it is always represented by a symbol Z.
all the baryonic matter of the universe not only earth is made up of chemical elements. since 118 elements in total have been discovered or identified yet from which 94 are naturally occurring elements and are found naturally on earth. the other 24 elements are called artificial or synthetic elements which are commonly produced in nuclear reactors.
what are some Examples of Elements?
gold and aluminum are two common examples of elements. imagine, you are holding a piece of Gold for your bridal in your hand. so, it is said that you are holding an element in your hands. however, we are providing you some examples of elements to clear the idea.
- Calcium
- Hydrogen
- Oxygen
- Boron
- Chloride
- Carbon
- Aluminum
- Neon
- Silicon
- Magnesium
What are Atoms?
atom is considered the smallest unit of ordinary or organic matter. all the chemical elements are made up of atoms. all the forms of matter like liquid, solid, gases, and plasma, etc, are composed of atoms. these atoms can be neutral or ionized. the average size of an atom is 100 picometer which hence atom is considered fairly small enough.
due to the smallness of atoms, classical physics always try to propose a predicting value of the behavior of atom but not the size.
every atom consists of a nucleus that is bounded around with electrons. so, the nucleus is consists of a number of protons and electrons surrounding it around. only oxygen atoms do not have neutrons. the 99.94% mass of the atom is present in the nucleus. the protons are neutral in electric charge while electrons have a negative electric charge on them.
when the number of protons and electrons have become equal, the atom is said to be electrically neutral. if this number in atom varies on either side, it has a negative or positive charge overall respectively. hence, this atom is said to be an ion. either positive ion or negative ion.
What is the Structure of the Atom?
there are few particles floating around the atoms. these particles are called subatomic particles of the atom. there are three fundamental particles such as an electron, proton, and neutron. the proton and neutron are part of the nucleus of the atom and electrons are revolving around the nucleus into different orbitals. however, these particles have some kind of charge on them as given below:
- neutrons are neutral particles having no charge on them.
- electrons are negatively charged particles.
- Protons are positively charged particles.
Difference Between Elements And Atoms in Tabular Form | |
Atoms | Elements |
An atom is the smallest particle present in an element that cannot be further subdivided. | An element is a pure and finest form of substance that cannot be converted to any simplest substance by using any physical or chemical methods or means. |
A nucleus is present consisting of protons, neutrons, and electrons. | Made with only a single kind of atoms. |
It is the smallest repeating unit of elements that make up all the matter in the universe. | It is a species in atoms. |
There are different species of elements in atoms in the periodic table. | The periodic table has only know species of elements which are also species of atoms. |
Oxygen is a common example of an atom that has 8 protons in its nucleus.
| All atoms that belong to chemical elements are examples of elements like oxygen that have 8 protons in their nucleus. |
Elements are combined together with the help of chemical reactions to form molecules. | atoms are combined to form molecules and if all molecules are made up of the same atoms then these molecules are called elements. |
elements are bigger than atoms and heavier in mass as compared to atoms. | they are tiny particles that cannot be seen even through microscopes. |
there are 118 elements are identified in total from which 94 are naturally occurring and 24 are synthetic elements. | there are 92 kinds of atoms in nature are found yet. |
the term element is often used in the periodic table and general chemistry. | atoms are often used in physics and atomic size or atomic mass is a very common term in chemistry. |
You May Also Like: