What is the Difference Between Element And Compound?
The Major Difference Between Element And Compound is that An Element is a substance that can not be broken down by chemical means and always contains similar kinds of atoms while A compound is a chemical species and is formed when two or more atoms joined together by a chemical mean.
What is an Element?
Element is the simplest form of the matter. all the substances in nature can be considered elements or compounds. all substances found on the periodic table are elements. it is species of an atom which have the same number of protons in their nuclei. the atomic number is denoted by Z.
to elaborate the atomic number in more detail let us have to set an example of oxygen. the atomic number of oxygen is 8 because the number of protons present in the nuclei of hydrogen is 8. so that, oxygen is symbolized for all those atoms which have the same number of protons.
there are one hundred and eighteen elements that are found on the earth till nowadays. from which 94 are the naturally occurring elements on the earth while the other 24 are artificially created by the human through atomic reactors or particle accelerators. they are known as synthetic elements.
What is a Compound?
like element, the compound is also a chemical substance that is formed by the composition of two or more than two atoms together by chemical bonds.they are referred to as heteroatomic molecules. compounds are of four types i.e. ionic compounds, covalent compounds, coordinate covalent compounds, and metallic compounds.
compounds are held together with the help of different chemical bonding and forces. the differences of bonds depending on the type of elements from which they are made up. different compounds of various compositions can interact with each other via a chemical reaction.
by doing this, the bonds between the chemical compounds are broken down and a new type of compound is created. the resulting compound would be a unique compound having different properties than that of the reaction compound.
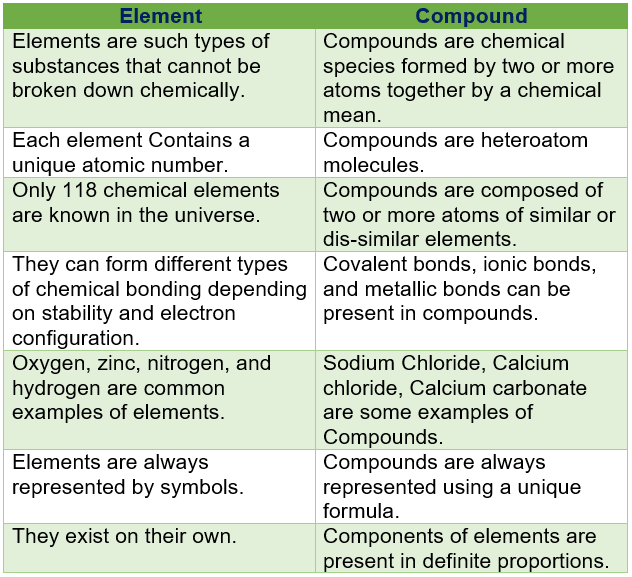
Difference Between Element And Compound in Tabular Form
| Element | Compound |
| elements are such types of substances that cannot be broken down chemically. | Compounds are chemical species formed by two or more atoms together by a chemical mean. |
| Each element Contains a unique atomic number. | compounds are heteroatomic molecules. |
| only 118 chemical elements are known in the universe. | compounds are composed of two or more atoms of similar or dis-similar elements. |
| they can form different types of chemical bonding depending on stability and electron configuration. | covalent bonds, ionic bonds, and metallic bonds can be present in compounds. |
| oxygen, zinc, nitrogen, and hydrogen are common examples of elements. | Sodium Chloride, Calcium chloride, Calcium carbonate are some examples of Compounds. |
| elements are always represented by symbols. | Compounds are always represented using a unique formula. |
| they exist on their own. | components of elements are present in definite proportions. |
You May Also Like: