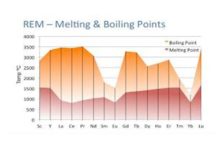
What is the Difference Between Cell and Battery?
The Major Difference Between Cell and Battery is that A cell is a unit of positive and negative electrodes. it is a device that converts the chemical energy into electrical energy while the battery is a combination of two or more cells makes a battery, used in cars, UPS, Emergency Lights, etc.
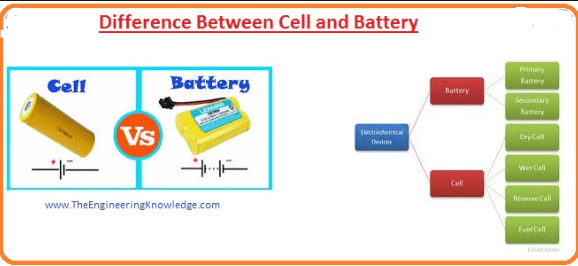
Explanation
A Cell is a device that uses chemical energy to produce or converts into electrical energy, it does not lose energy over time if it is not in use. It is a practical way of storing energy, since it does not lose energy over time, which is why it is widely used, especially in household appliances.
A battery converts chemical energy into electrical energy, but it will lose energy over time if it is not recharged. so, it needs continuous recharging so as not to lose its charge even if it is not in use. batteries can have domestic and industrial use.
Difference Between Cell and Battery in Tabular Form
Cell | Battery |
Batteries hold their charge for long periods of time. | Cells lose their charge over time, even if they are not in use. |
A battery is a primary generator. | A Cell is a secondary generator. |
The battery stores the potential to generate electrical energy through chemical energy. | The Cell receives electrons from an external generator and stores them for later use. |
Batteries have a single electrolytic cell. | The Cell has one or more electrolytic cells. |
Stacks are limited by their size. | Cells are limited by the number of charges they can handle. |
The batteries have a voltage that goes from 1.5 v to 9 v. | Cells have a voltage that goes from 2 v to 14.8 v. |
do not store energy | stores energy |
Alkaline and Alkaline manganese are common types of cells. | Nickel-cadmium, Lead-acid, Nickel-metal hydride, Polymer-lithium, and Lithium-ion are common types of batteries. |
cells are commonly used in flashlights, toys, pacemakers, calculators. | Batteries are commonly used in cars, mobiles, electric cars. |
What is the Battery?
It is a type of secondary energy generator, this means that it needs a primary device to transform the energy. It is made up of electrochemical cells that have an electrolyte and two electrodes, one positive and the other negative. They are also known as accumulators.
Batteries lose their charge constantly, no matter if they are used or not, however, it is a reversible process, because they can be recharged. In addition, they work by accumulating the electrical charge that has been produced by a primary generator. Its voltage ranges from 2 V to 14.8 V.
To function, batteries use a process of oxidation and reduction, one electrode loses electrons with oxidation and the other receives them. This process is reversed by passing an electric current with a generator, this starts a new charging cycle.
Its origin dates back to the late 19th century when Waldermar Jungner, a Swedish scientist, invented a battery with nickel, cadmium, and a calcium hydroxide electrolyte. By 1940 this battery began to be commercialized in the USA and it was Thomas Alva Edison who created the model that is commercialized today based on the idea of Waldermar.
A battery is a secondary generator of energy, composed of one or more electrochemical cells. Each of these cells has its positive and negative electrodes and an electrolyte.
It is a secondary generator because it requires a primary device to transform energy.
Batteries are also known as accumulators.
Origin of battery
After Alessandro Volta invented the voltaic pile in 1800, other researchers and scientists followed who tried to improve on what he had shown.
One of them was Gastón Planté, a French scientist who created a lead-acid battery in 1860, which although it was not well received at first, it did have a great relevance with the rise of electricity at the end of the 19th century.
It was precisely at this time that the Swedish scientist Waldermar Jungner invented a battery with nickel and cadmium electrodes and potassium hydroxide electrolyte. His model was so successful that it began to be produced and marketed in the United States in the late 1940s.
American inventor Thomas Alva Edison, the creator of the incandescent light bulb, took Jungner’s idea and created his own battery, which is still being produced and marketed today.
In the seventies, when the first lithium batteries began to be sold, which today are very popular because they are used for the operation of electric cars.
characteristics of Battery
Batteries have characteristics in terms of their process of loss, recharge, and accumulation of energy.
- A battery will lose its electrical charge gradually, regardless of whether it is used or not.
- The loss of charge of a battery is a reversible process, which gives them a much longer useful life compared to batteries.
- Batteries accumulate an electrical charge that has previously been produced with a primary generator.
- The voltage of a battery ranges from 2 volts (in lead batteries) to 14.8 volts in some lithium polymer batteries.
Operation of a Battery
Batteries work through a process of reduction and oxidation, similar to that of a battery. In this sense, one of the electrodes loses electrons with oxidation, while the other electrode is reduced and gains electrons.
In the case of batteries, this process can be reversed by applying an electrical current to return the device to its original state, initiating a new charge cycle.
Types of Batteries
Based on their chemical components, batteries can be classified into five types of battery:
- Nickel-cadmium batteries.
- Nickel-metal hydride batteries.
- Lithium-ion batteries.
- Polymer-lithium batteries.
- Lead-acid batteries.
Nickel-cadmium batteries
The positive electrode is nickel hydroxide and the negative electrode is cadmium, while the electrolyte is potassium hydroxide. They accept high voltages and overloads, but their energy density is very low, coupled with the fact that cadmium is a highly toxic element. They have domestic and industrial use.
Nickel-metal hydride batteries
Its negative electrode is nickel and the positive electrode is a metal hydride alloy. Their energy density is higher, but they do not work properly at low temperatures. They are the batteries used in electrically powered vehicles.
Lithium-ion batteries
They have a negative electrode of graphite and a positive electrode of cobalt oxide or manganese oxide. Their development is recent, they have high energy densities and can be recharged without the need for them to be completely discharged. However, they do not allow temperature changes.
They are the type of battery used by e-book readers and mobiles.
Polymer lithium batteries
They are similar to lithium-ion batteries, but with a higher energy density. They are expensive and run the risk of exploding from overheating.
They are used in mobile phones and photographic equipment.
Lead-acid batteries
It is composed of two lead electrodes and a sulfuric acid dissolution electrolyte. Being lead, they are very heavy, and therefore impractical. Their useful life is limited since they do not support deep discharges or overloads, they are highly polluting and their energy potential is very low; hence they are the cheapest on the market.
What is Cell?
A battery is a primary generator of electrical energy (it generates energy by itself), composed of an electrolytic cell, two metal electrodes (one positive, called the cathode, and one negative, called the anode), and a liquid or pasty medium called the electrolyte.
The energy stored in the battery is not lost over time; that’s why they can be kept for a few years and will still work. However, its internal components do degrade over time, causing the battery to deteriorate and cannot be used.
It is a type of primary energy generator, that is, it generates energy by itself. It has an electrolytic cell and two electrodes, one positive and one negative, in a viscous or liquid medium known as an electrolyte.
Their energy is not lost over time, they can be stored for years. However, its components can degrade and make battery use impossible.
Batteries work when the battery electrodes react with the electrolyte gel or paste, this oxidizes the negative electrode and generates electrons. In turn, the cathode or positive electrode is reduced, losing electrons. Electrons from the negative pole pass to the positive pole through a conductor, thus generating electricity.
The battery was invented by Alessandro Volta in 1800 and it was baptized as the voltaic battery. His discovery led to a method of storing energy and showed that by grouping batteries in series, the voltage increased, a great discovery for electricity.
Over time, many European scientists tried to improve on Volta’s invention, but it was not until 1868 that French scientist and engineer Georges Leclanché created the pile we all know. This battery had two zinc and carbon electrodes immersed in an ammonium chloride solution, this mixture was sealed with manganese dioxide and carbon powder.
With the passage of time, this battery evolved into a safer model: dry batteries, in which you have a zinc cylinder, a carbon bar, and an electrolytic filling inside a sealed cylinder. Difference Between Cell and Battery
The energy of a battery is not infinite, it depends on the electrons. Batteries are also known as perfect voltage sources, this means that their internal resistance is zero, but as they wear down, resistance develops and the voltage is reduced.
Batteries can have voltages ranging from 1.5 volts to 9 volts.
Batteries can be regular, alkaline, or alkaline manganese.
Origin of Cells
The first pile was created by Alessandro Volta in 1800 and was called the voltaic pile. Although its presentation was far from the current industrialized batteries, its creation allowed not only to discover a form of energy storage but also to verify that if several batteries were connected in series, it was possible to increase the voltage at will, which was a discovery.
As a result of the creation of Volta, many experiments arose, especially in Europe, to improve this invention. However, the one that gave rise to the pile that we know today is the Leclaché pile, the result of the research of Georges Leclanché, a French scientist and engineer. Difference Between Cell and Battery
This battery, invented in 1868, was composed of two zinc and carbon electrodes, immersed in a solution of ammonium chloride. A paste of manganese dioxide surrounded the carbon electrode, while the carbon powder acted as a depolarizer.
With the Leclanché battery, a more efficient way of storing energy was discovered: dry batteries. They consist of a zinc cylinder, which is the negative pole, a carbon rod in the center, which acts as the positive pole, and an electrolytic filler. The cylinder is sealed, preventing leakage of toxic compounds.
Today, dry cells are produced industrially and are essential for the operation of many devices in everyday use.
Operation of a cell
The battery electrodes react to the electrolyte paste or gel, which generates an oxidation process at the anode (negative electrode) that activates the production of electrons. For its part, in the cathode (positive electrode), a reduction process is generated that causes a deficit of electrons.
When excess electrons from the negative electrode pass to the positive electrode through an outer conductor, an electric current is generated. Difference Between Cell and Battery
characteristics of Cell
Batteries have a number of characteristics that influence the generation of energy and its duration.
- The series connection of a set of batteries allows multiplying the electrical voltage at will.
- The energy of a battery is not infinite, it is limited by the size of the electrons and the distance between them.
- The batteries are made up of a perfect voltage source, which means that their internal resistance is zero. As the battery wears out or deteriorates, the resistance begins to increase, and the greater the resistance, the lower the voltage. Therefore, the energy will be more and more insufficient.
- The components of a battery are sensitive to changes in temperature, hence very high or very low temperatures can affect the operation of the device.
- The current battery voltage ranges from 1.5 volts (for a typical low-priced battery) to 9 volts.
Types of Cells
Depending on their electrochemical characteristics, batteries can be ordinary, alkaline, or alkaline manganese.
Common batteries
They are dry cells made up of a zinc cylinder (negative pole), an ammonium chloride electrolyte paste, and a carbon bar in the center (positive pole).
They are the cheapest batteries on the market, so they are usually included in many new devices.
Alkaline batteries
They are similar to common batteries, except for the electrolyte paste that contains potassium or sodium chloride, the inside of the zinc cylinder, which is rough. This serves to create a larger contact surface.
Alkaline batteries last longer than a regular battery and the current is more stable. In addition, they operate at a higher temperature range than a typical battery.
Alkaline Manganese Batteries
They are alkaline batteries whose positive pole is made of zinc or lithium, while the negative pole is made of manganese dioxide or silver oxide. They are widely used in precision devices, such as watches, pacemakers, or calculators. Difference Between Cell and Battery.
Values to consider in cells and batteries
- Ampere. Most of the current provided by the battery will depend on the load to which it is subjected, although it will also be limited by the internal resistance of the battery/cell, which can be quantified by means of an amperemeter.
- Capacity. This refers to the current that the battery can provide in a period of time whose measurement is quantified in ampere-hours (Ah). For smaller capacity batteries, milliampere-hours (mAh) are used. An example: if a battery has a capacity of 4 Ah, this means that, theoretically, the battery could provide a current of 1 A for 4 hours or 0.2 A for 20 hours.
Of course, if very large currents are demanded it is likely that the battery could provide that amount in a shorter time, but it is very possible that the battery simply cannot provide those high currents and, if it were to do so, it would overheat and be damaged by such demand. That is why these considerations must be borne in mind and let’s not expect a small device to give more than it can actually give.
There is a mathematical expression that can quantify the time a battery can provide the desired current. This expression is:
T = H * (AhM / [I * H] ^ n)
In this expression, AhM is the battery capacity in ampere-hours -manufacturer data-, I is the current the battery is expected to provide, n is the Peukert number -this data is provided by the manufacturer and this number is an estimation factor to obtain a more realistic value of T with high currents- and H is the duration in hours in which the battery was tested by the manufacturer
-this number is also usually provided by most manufacturers and is usually 20 hours- Additionally, the Peukert number increases as time passes as the battery also degrades.
- Voltage.
Battery or cell connections
When using several cells or batteries and assuming that they are all of the same brands and with the same characteristics, the two ways to connect them are in series or in parallel.
When connected in series, the total voltage is equal to the sum of the voltage of each one while the total capacity is equal to the capacity of a single piece -battery or cell-
On the other hand, when the cells/batteries are connected in parallel, the total voltage of the array is equal to the voltage of a single cell/battery while the total capacity is equal to the sum of the capacity of each cell/battery.
Cautions with the handling and connections of cells and batteries
It is important to remember that you should never connect the terminals of the cells/batteries directly because, having a very low internal resistivity, the current flow is very large which causes the cell/battery to overheat, damage, and even explode.
Regarding rechargeable batteries, an appropriate charger must be used for each type of battery and the batteries must be connected to the corresponding polarity.
In a series arrangement of batteries, it must be ensured that all the batteries are in good condition since if one of them is discharged, then this battery will act as one more load, in addition to that this voltage will be inverse to that supplied by the battery. good battery, causing damage to the good battery and the load.
This phenomenon is known as reversible loading.
The topics related to batteries are extensive, so mentioning and giving details of each type of battery would be too much for this space, so it would be better to talk about each type separately. As additional data is found that can enrich this topic.
the theme of this blog will be updated and improved, so for now some sites are recommended. For example, this site contains good and detailed information about lithium-ion batteries:
You May Also Like: